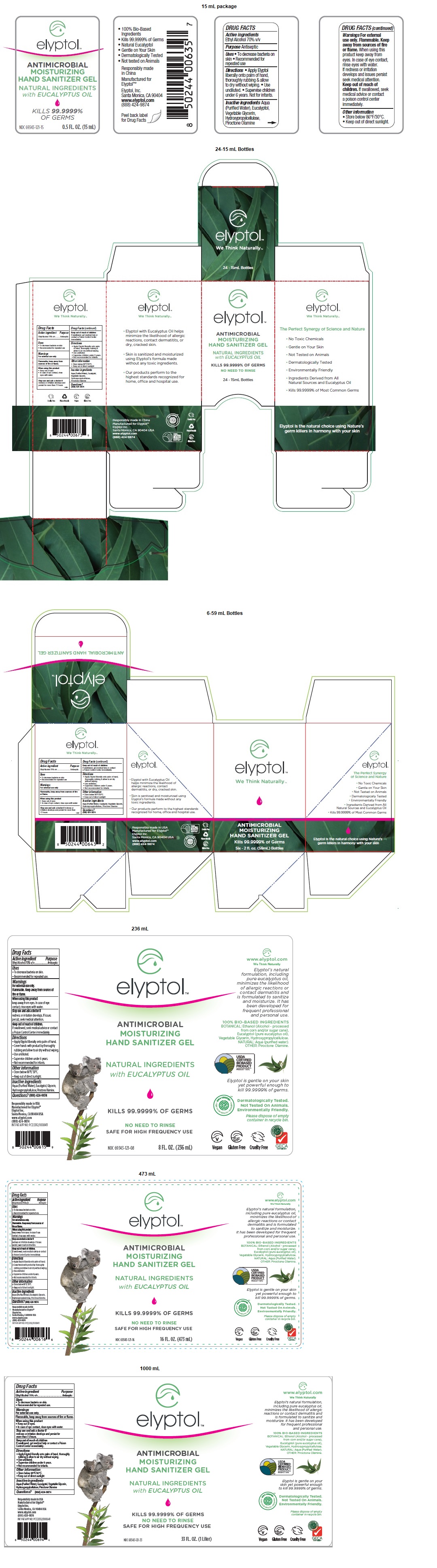
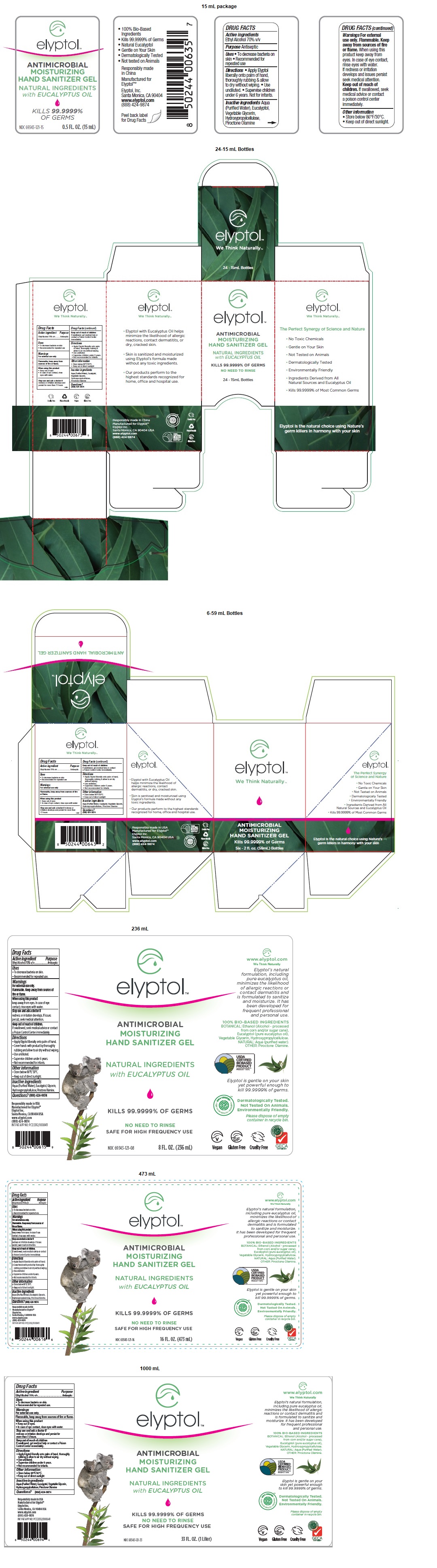
Label: ELYPTOL ANTIMICROBIAL HAND GEL- ethanol gel
-
NDC Code(s):
69343-121-02,
69343-121-03,
69343-121-08,
69343-121-15, view more69343-121-16, 69343-121-33, 69343-121-34, 69343-121-42, 69343-121-62
- Packager: Elyptol Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
ANTIMICROBIAL
MOISTURIZING
HAND SANITIZER GELNATURAL INGREDIENTS
with EUCALYPTUS OIL
KILLS 99.9999% OF GERMS
NO NEED TO RINSE
SAFE FOR HIGH FREQUENCY USE
We Think Naturally™
The Perfect Synergy of Science and Nature
• No Toxic Chemicals
• Gentle on Your Skin
• Not Tested on Animals
• Dermatologically Tested
• Environmentally Friendly
• Ingredients Derived from All Natural Sources and Eucalyptus Oil
• Kills 99.9999% of Most Common Germs
Elyptol is the natural choice using Nature’s germ killers in harmony with your skin
• Elyptol with Eucalyptus Oil helps minimize the likelihood of allergic reactions, contact dermatitis, or dry, cracked skin.
• Skin is sanitized and moisturized using Elyptol’s formula made without any toxic ingredients.
• Our products perform to the highest standards recognized for home, office and hospital use.
100% BIO-BASED INGREDIENTS
BOTANICAL: Ethanol (Alcohol - processed from corn and/or sugar cane), Eucalyptol (pure eucalyptus oil), Vegetable Glycerin, Hydroxypropylcellulose.
NATURAL: Aqua (Purified Water).
OTHER: Piroctone Olamine.Elyptol is gentle on your skin yet powerful enough to kill 99.9999% of germs.
Dermatologically Tested.
Not Tested On Animals.
Environmentally FriendlyPlease dispose of empty container in recycle bin.
Responsibly made in USA
Manufactured for Elyptol™
Elyptol Inc.
Santa Monica, CA 90404 USA
www.elyptol.com
(888) 424-9874Cruelty Free
Please Recycle
Vegan
Gluten Free
EWG VERIFIED • EWG.ORG • FOR YOUR HEALTH
- Packaging
-
INGREDIENTS AND APPEARANCE
ELYPTOL ANTIMICROBIAL HAND GEL
ethanol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69343-121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 7 mL in 10 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EUCALYPTOL (UNII: RV6J6604TK) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69343-121-15 24 in 1 CARTON 11/13/2020 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69343-121-02 1 in 1 CARTON 11/01/2014 2 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69343-121-62 6 in 1 CARTON 11/13/2020 3 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:69343-121-03 1 in 1 CARTON 11/01/2014 4 89 mL in 1 PACKAGE; Type 0: Not a Combination Product 5 NDC:69343-121-08 1 in 1 CARTON 11/01/2014 5 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:69343-121-16 1 in 1 CARTON 11/01/2014 6 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:69343-121-33 1 in 1 CARTON 11/13/2020 7 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:69343-121-34 1 in 1 CARTON 11/01/2014 8 1005 mL in 1 BOTTLE; Type 0: Not a Combination Product 9 NDC:69343-121-42 1 in 1 CARTON 11/13/2020 9 1250 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/01/2014 Labeler - Elyptol Inc. (079594781) Establishment Name Address ID/FEI Business Operations 220 Laboratories LLC. 783247950 manufacture(69343-121)