Label: CREAM cream
- NDC Code(s): 84067-331-01
- Packager: Shantou Youjia E-Commerce Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
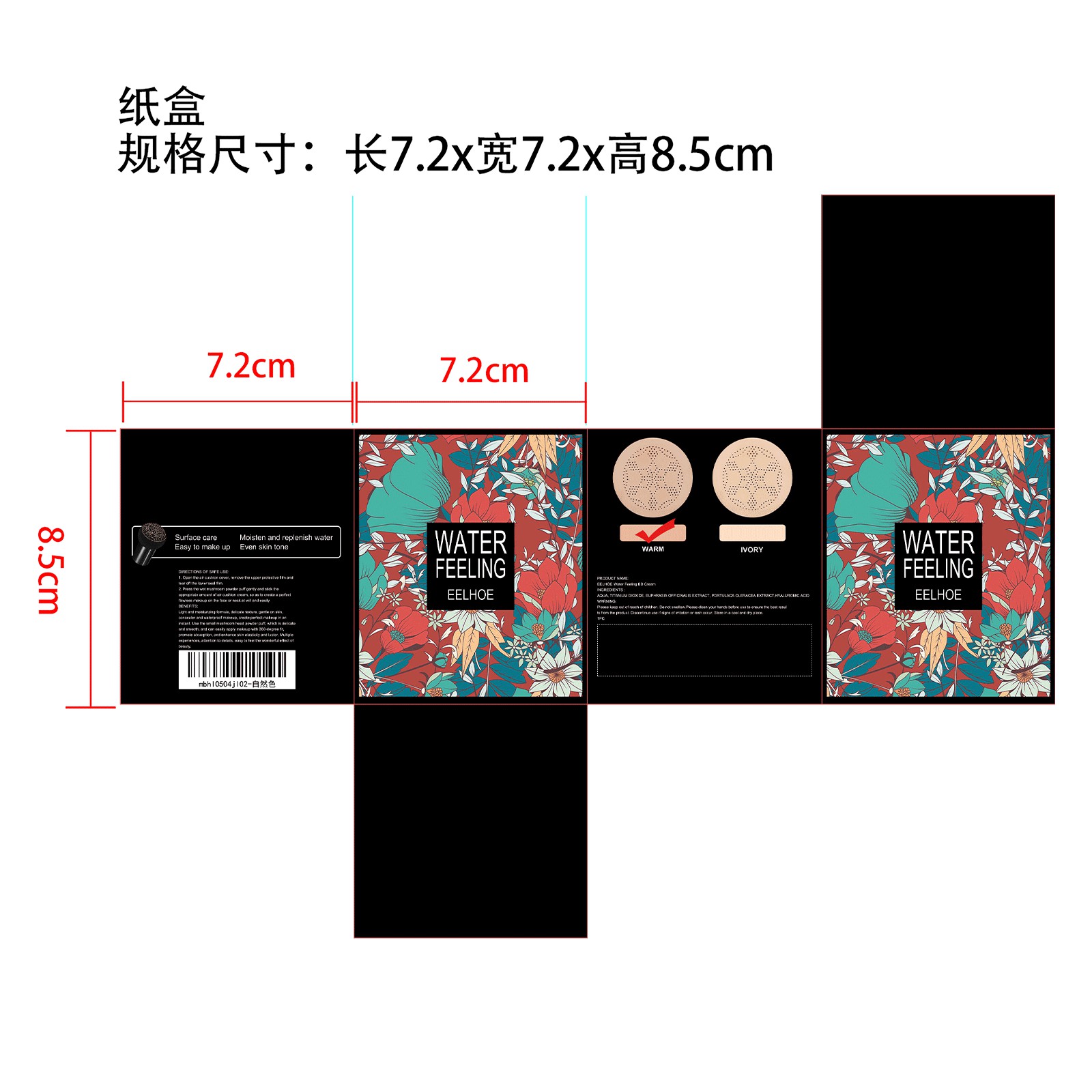
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CREAM
cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84067-331 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 34.5 g in 115 g HYALURONIC ACID (UNII: S270N0TRQY) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONIC ACID 23 g in 115 g PURSLANE (UNII: M6S840WXG5) (PURSLANE - UNII:M6S840WXG5) PURSLANE 11.5 g in 115 g EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 17.25 g in 115 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 28.75 mL in 115 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84067-331-01 115 g in 1 BOX, UNIT-DOSE; Type 0: Not a Combination Product 02/01/2024 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 02/01/2024 12/31/2024 Labeler - Shantou Youjia E-Commerce Co., Ltd. (711173127) Establishment Name Address ID/FEI Business Operations Shantou Youjia E-Commerce Co., Ltd. 711173127 label(84067-331)