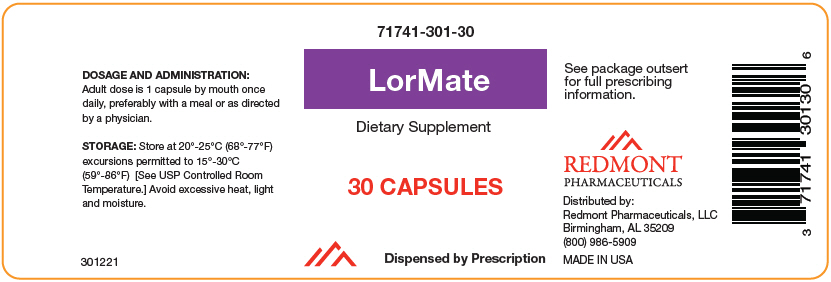
Label: LORMATE- levomefolate calcium, methylcobalamin, and turmeric capsule
- NHRIC Code(s): 71741-301-30
- Packager: Redmont Pharmacetuicals, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated May 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HEALTH CLAIM
-
INDICATIONS
LorMate is an orally administered dietary supplement intended for the clinical dietary management of suboptimal nutritional status in patients taking methotrexate to treat their chronic inflammatory diseases where advanced folate, Vitamin B-12 supplementation and maintenance of good health is needed under physician supervision.
-
DIETARY SUPPLEMENT
LorMate has been formulated, manufactured and labeled in accordance with the requirements for Dietary Supplements. LorMate is to be used only under a physician's supervision.
Supplement Facts Serving size: 1 Capsule
Serving per Container: 30Amount per serving Daily Value Percent Daily Values are based on a 2,000 calorie diet. Your daily values may be higher or lower depending on your calorie needs. - *
- Daily Value not established
Folate (as L-5-Methyltetrahydrofolic acid, Calcium salt) 1 mg
(1,670 mcg DFE)250% Vitamin B-12 (as Methylcobalamin) 1 mg 16667% CurcuGen™-Turmeric (Curcuma longa) (rhizome) Extract (standardized to 50% Total Curcuminoids) & Essential Oil (Oleoresin) Complex 500 mg * -
DESCRIPTION
LorMate consists of a mixture of L-5-methyltetrahydrofolic acid, Calcium salt – 1mg, Vitamin B-12 as methylcobalamin – 1 mg, and CurcuGen™ Turmeric Extract and Essential Oil Complex – 500mg. L-5-methyltetrahydrofolate provides the methotrexate-induced distinctive nutritional requirement for folate due to competitive inhibition of reduced folate production in certain individuals who may have a more pronounced genetic polymorphism in the folate metabolic pathway. Methylcobalamin provides adequate Vitamin B-12 stores to avoid masking borderline deficiency Vitamin B-12 with external folate supplementation. Curcumin provides an increased nutritional requirement for antioxidants associated with methotrexate induced oxidative stress.
OTHER GRAS INGREDIENTS: Vegetable Cellulose (Capsule), Microcrystalline Cellulose, Silicon Dioxide, Magnesium Stearate.
All ingredients in LorMate are GRAS ingredients, approved food additives, or approved color additives.
Allergen: NONE
-
METHOTREXATE-INDUCED DISTINCT NUTRITIONAL REQUIREMENTS
Methotrexate is the most widely used disease modifying drug in the treatment of inflammatory diseases such as rheumatoid arthritis, psoriatic arthritis or psoriasis. Despite its therapeutic benefit as many as 30% of patients discontinue or reduce their dose due to its adverse effects caused by the failure to compensate for the increased need for folate acid. The increased requirement for folate results from the methotrexate induced reduction in the folate-mediated one-carbon metabolic pathway and other enzymatic processes for which reduced folate is a cofactor.
Methylcobalamin provides adequate Vitamin B-12 stores to avoid masking borderline deficiency of Vitamin B-12 with exogenous folate supplementation.
Methotrexate adverse events are thought to be caused by a disruption of cellular redox homeostasis (severe oxidative stress) with depletion of natural antioxidant mechanisms and marked rise in oxidative by-products. This creates increased nutritional requirement for supplemental dietary antioxidants (curcuminoids) thus reducing oxidative burden.
-
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
L-5-methyltetrahydrofolate
L-5-methyltetrahydrofolate, the bioactive form of folate is well absorbed and able to participate in all biochemical processes for which folate is a cofactor.
Cobalamin
Cobalamin (Vitamin B-12) is well absorbed under normal conditions. However, geriatrics and individuals with reduced stomach acid production may inadequately absorb cobalamin. This defect can be corrected with administration of supplemental dietary Vitamin B-12. Individuals with autoimmune atrophic gastritis should be administered supplemental parenteral Vitamin B-12.
Mechanism of Action
L-5-methyltetrahydrofolate
Folic acid and its derivatives address the unique nutritional requirement for folic acid of patients using methotrexate to treat certain inflammatory diseases including rheumatoid arthritis, psoriatic arthritis or psoriasis. Folic acid and its derivatives are essential for the synthesis of DNA, the modification of DNA and RNA, the synthesis of methionine from homocysteine, serine and various other chemical reactions involved in cellular metabolism. These reactions are important in the metabolism and biosynthesis of amino acids, nucleic acids, membrane lipids, cholesterol and neurotransmitters. These reactions are collectively known as folate-mediated one-carbon metabolism. A significant proportion of the general population, however, has one or another polymorphism (genetic variation) in the folic acid metabolic pathway. Supplementing the folic acid metabolic pathway with L-5-methyltetrahydrofolate salts therefore may obviate or reduce the effect of these polymorphisms
Methylcobalamin
Methylcobalamin is an essential cofactor in many folate dependent metabolic pathways. Minor deficiencies in cobalamin levels may be obscured in the presence of greater than normal folate levels. Although this may suppress such clinical signs as megaloblastic anemia, it does not prevent neurological damage associated with Vitamin B-12 deficiency. Cobalamin supplementation provides sufficient Vitamin B-12 to assure that L-5-methyltetrahydrofolate administration does not mask cobalamin deficiency.
Curcuminoids
The pharmacological actions of curcumin have been studied by various researchers worldwide. Curcumin has the ability to suppress acute and chronic inflammation. It reduces inflammation by lowering histamine levels and by possibly increasing the production of natural cortisone by adrenal glands. In in vitro studies of curcumin demonstrated anti-inflammatory action on human vascular cells. The mechanism of action of curcumin anti-inflammatory effect is by attenuating inflammatory response of TNF-α stimulated human endothelial cells by interfering with NF-κB. Additionally curcumin is capable of preventing platelet-derived growth factor (PDGF).
-
TOXICITY
L-5-methyltetrahydrofolate
No toxicity issues have been reported from folate supplementation. However, folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B-12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Curcuminoids
No significant curcumin toxicity has been reported. Acute doses up to 12 gm and chronic doses of 10 gm/d have been found to be safe. Although dozens of human clinical trials have consistently demonstrated the lack of toxicity from curcumin, minor gastrointestinal complaints have occasionally been noted in these trials. A few in vitro studies suggest that curcumin may interfere with platelet aggregation.
- CONTRAINDICATIONS
-
WARNINGS
Folate may interfere with absorption of certain medications. Patients receiving both LorMate and tetracycline should take these medications at least one hour apart. The serum levels and the utilization of folate may be reduced when taken with sulfasalazine, phenytoin, statins and some birth control medications.
-
PRECAUTIONS
LorMate should only be used under the direction and supervision of a licensed medical practitioner.
LorMate use should be under the supervision of a physician when administered to patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking other medications.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B-12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Bottles of 30 capsules (71741-301-301).
LorMate is supplied as a yellowish powder in a clear transparent capsule.
- 1
- Redmont Pharmaceuticals does not represent this product code to be National Drug Code (NDC). Product code is formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
- STORAGE
-
HEALTH CLAIM
This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking Vitamin B-12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (64 FR 8760). 1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription. This is not an Orange Book product. This product may be administered only under a physician's supervision and all prescriptions using this product shall be pursuant to state statues as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760)TAMPER EVIDENT: Do not use if seal is broken or missing.
MADE IN USA
Distributed by:
Redmont Pharmaceuticals, LLC
Birmingham, AL 35209
800-986-5909CurcuGen™ is a registered trademark of Dolcas BioTech LLC
CurcuGen™ Turmeric Extract - Patents PendingThese statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
301321
- PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
LORMATE
levomefolate calcium, methylcobalamin, and turmeric capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:71741-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 1 mg Methylcobalamin (UNII: BR1SN1JS2W) (Methylcobalamin - UNII:BR1SN1JS2W) Methylcobalamin 1 mg Turmeric (UNII: 856YO1Z64F) (Turmeric - UNII:856YO1Z64F) Turmeric 500 mg Inactive Ingredients Ingredient Name Strength Microcrystalline Cellulose 102 (UNII: PNR0YF693Y) Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Palmitostearate (UNII: R4OXA9G5BV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71741-301-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 03/23/2020 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 22 mm Labeler - Redmont Pharmacetuicals, LLC (080843607)