Label: NASONEX- mometasone furoate spray, metered
-
NDC Code(s):
0113-1720-01,
0113-1720-02,
0113-1720-03,
0113-1720-04, view more0113-1720-05, 0113-1720-06, 0113-1720-07, 0113-1720-11
- Packager: L. Perrigo Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
Only for use in the nose. Do not spray into your eyes or mouth.
Do not use
- •
- in children under 2 years of age
- •
- to treat asthma
- •
- if you have an injury or surgery to your nose that is not fully healed
- •
- if you have ever had an allergic reaction to this product or any of the ingredients
Ask a doctor or pharmacist before use if you are taking
- •
- medicine for HIV infection (such as ritonavir)
- •
- a steroid medicine for asthma, allergies or skin rash
- •
- ketoconazole pills (medicine for fungal infection)
When using this product
- •
- the growth rate of some children may be slower
- •
- stinging may occur for a few seconds right after use
- •
- you may start to feel relief within 12 hours and full effect after several days of regular, once-a-day use
- •
- do not share this bottle with anyone else as this may spread germs
- •
- remember to tell your doctor about all the medicines you take, including this one
Stop use and ask a doctor if
- •
- you have, or come into contact with someone who has chickenpox, measles or tuberculosis
- •
- your symptoms do not get better within 7 days of starting use or you get new symptoms such as severe facial pain or thick nasal discharge. You may have something more than allergies, such as an infection.
- •
- you get a constant whistling sound from your nose. This may be a sign of damage inside your nose.
- •
- you get an allergic reaction to this product. Seek medical help right away.
- •
- you get new changes to your vision that develop after starting this product
- •
- you have severe or frequent nosebleeds
-
Directions
Read insert (inside package) for how to:
- •
- prime the bottle
- •
- use the spray
- •
- clean the nozzle
- •
- shake well before each use
- •
- use this product only once a day
- •
- do not use more than directed
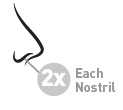
Adults & children 12 years of age and older
- •
- 2 sprays in each nostril once daily while sniffing gently
Children 2 to 11 years of age
- •
- the growth rate of some children may be slower while using this product. Children should use for the shortest amount of time necessary to achieve symptom relief. Talk to your child’s doctor if your child needs to use the spray for longer than two months a year.
- •
- an adult should supervise use
- •
- 1 spray in each nostril once daily while sniffing gently
Children under 2 years of age
- •
- do not use
- Other information
- Inactive Ingredients
- Questions or comments?
-
Consumer Information Leaflet
NASONEX®
24HR ALLERGY
72A00 SY J2
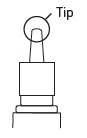
A - GETTING STARTED
NASONEX® 24HR Allergy works best at relieving your allergy symptoms when you get a full dose. Here’s how to get started, in five easy steps.
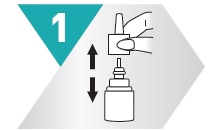
1 – SHAKE
Shake spray bottle well.
Remove cap.
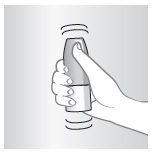
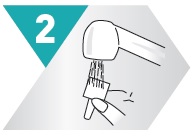
2 – PRIME
Do this when:
- •
- Starting a new bottle
- •
- Bottle has not been used in one week
- •
- Just cleaned nozzle
Otherwise go to step 3
Aim away from face. Grasp spray bottle as shown. Pump until fine mist appears. Pumped ten times and still no mist? Spray nozzle may be clogged. See KEEP IT CLEAN, other side.
NOTE: There is enough medicine in the bottle to allow for priming sprays plus the number of sprays labeled on the bottle.
3 – BLOW
Blow nose gently to clear nostrils.
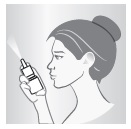
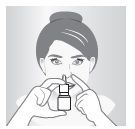
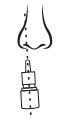
4 – AIM
Close one nostril and put tip of spray nozzle in other nostril.
Put just the tip into your nose.
Aim slightly away from center of nose.
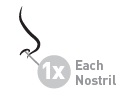
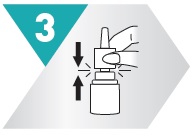
5 – BREATHE AND SPRAY
While sniffing gently, press down on spray nozzle once or twice (according to dosing instructions). You'll feel a light mist in your nose. Breathe out through your mouth. Repeat in other nostril.
Wipe spray nozzle with clean tissue and replace cap.
Dosing and Cleaning instructions
See other side
SIDE 1
B – DIRECTIONS FOR USE
NASONEX® 24HR Allergy works best when you use it daily. Here’s how to get started.
Children age 2-11
An adult should supervise use
Use one spray in each nostril once daily.
Check with your child’s doctor if child needs to use NASONEX for longer than two months a year.
WARNING:
Do not spray in your eyes. Only for use in your nose.
Users age 12 or older
Use two sprays in each nostril every day.
Other important information for use
- •
- Do not use more than directed.
- •
- If you forget a dose, do not double the next dose.
- •
- Do not spray into eyes or mouth.
- •
- If you get the spray in your eyes, rinse well with water.
- •
- If allergy symptoms do not improve after one week, stop using and talk to a doctor.
- •
- Do not share this bottle with anyone else as this may spread germs.
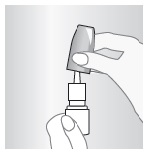
C – KEEP IT CLEAN
A clean spray nozzle helps ensure a full dose.
Clean it weekly, or if it's clogged. Don't try to unblock nozzle with pin or sharp object – that can damage it.
Remove spray nozzle by grasping at base and pulling up.
Rinse under running tap water, and dry at room temperature.
Aim away from your face and gently, but firmly, replace spray nozzle.
If spray nozzle is clogged, soak in warm water. Then repeat steps 2 and 3.
Questions or comments? Call toll-free 1-800-864-9649
Store between 20º-25ºC (68º-77ºF)
Nasonex, the Nasonex logo, the bottle and cap designs, and other design elements are trademarks of Organon LLC or its affiliates and used under license.
Distributed By
Perrigo®
Allegan, MI 49010
©2023. All rights reserved.
SIDE 2
-
Package/Label Principal Display Panel
PERRIGO Selfcare™
non-drowsy / scent-free mist
NASONEX®
24HR ALLERGY
MOMETASONE FUROATE NASAL SPRAY, 50 MCG/SPRAY ALLERGY SYMPTOM RELIEVER (GLUCOCORTICOID)*
ALLERGY + CONGESTION
FULL PRESCRIPTION STRENGTH
24HR RELIEF of:
nasal congestion / runny nose
sneezing / itchy nose
*Mometasone Furoate is a steroid medicine known as a glucocorticoid
30 SPRAYS
0.25 FL. OZ. (7.5 mL)
-
INGREDIENTS AND APPEARANCE
NASONEX
mometasone furoate spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0113-1720 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOMETASONE FUROATE MONOHYDRATE (UNII: MTW0WEG809) (MOMETASONE - UNII:8HR4QJ6DW8) MOMETASONE FUROATE 50 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0113-1720-01 1 in 1 PACKAGE 06/30/2025 1 30 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:0113-1720-11 1 in 1 PACKAGE 06/29/2022 2 60 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:0113-1720-02 1 in 1 PACKAGE 06/29/2022 3 120 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:0113-1720-03 1 in 1 PACKAGE 06/29/2022 06/29/2022 4 72 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 5 NDC:0113-1720-06 2 in 1 PACKAGE 06/29/2022 5 120 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 6 NDC:0113-1720-04 1 in 1 PACKAGE 06/29/2022 06/29/2022 6 144 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 7 NDC:0113-1720-05 2 in 1 PACKAGE 06/29/2022 06/29/2022 7 144 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 8 NDC:0113-1720-07 3 in 1 PACKAGE 01/21/2024 8 144 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215712 06/29/2022 Labeler - L. Perrigo Company (006013346)