Label: STAY AWAKE MAXIMUM STRENGTH- caffeine tablet, film coated
- NDC Code(s): 41163-434-11
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
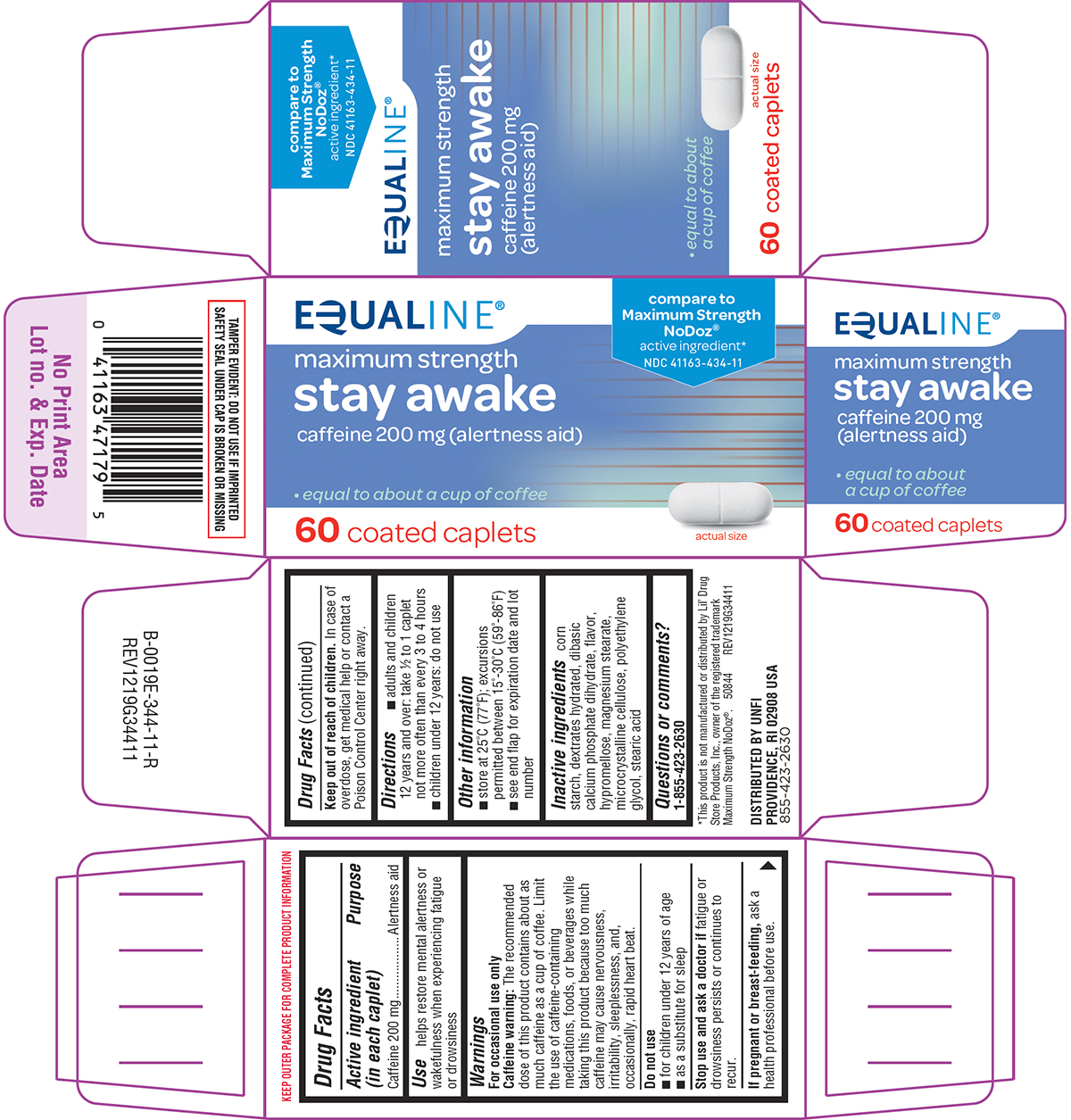
Principal display panel
EQUALINE®
compare to
Maximum Strength
NoDoz®
active ingredient*NDC 41163-434-11
maximum strength
stay awake
caffeine 200 mg (alertness aid)• equal to about a cup of coffee
60 coated caplets
actual size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed by Lil’ Drug
Store Products, Inc., owner of the registered trademark
Maximum Strength NoDoz®. 50844 REV1219G34411DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
855-423-2630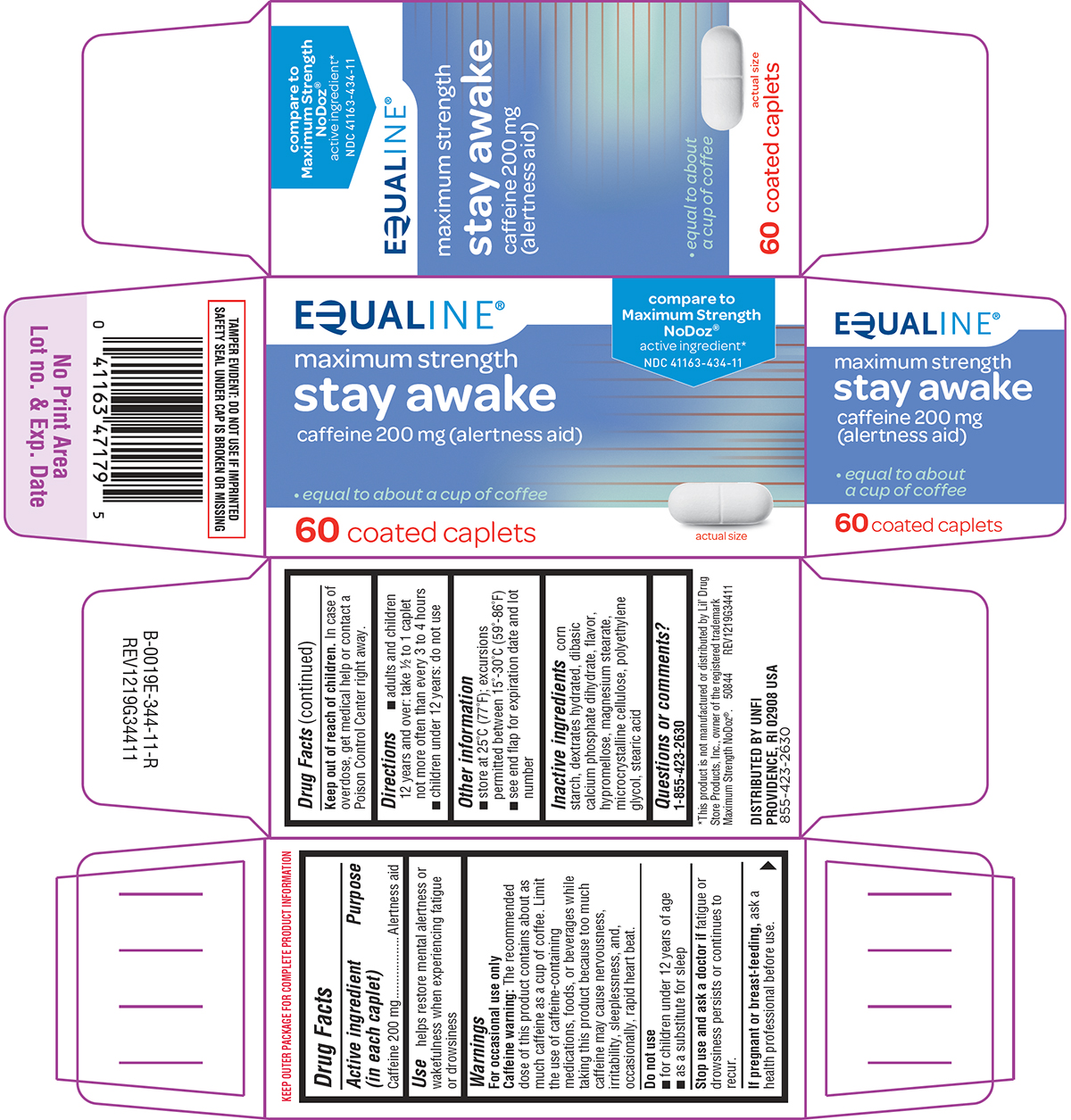
Equaline 44-344
-
INGREDIENTS AND APPEARANCE
STAY AWAKE MAXIMUM STRENGTH
caffeine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-434 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape OVAL Size 15mm Flavor PEPPERMINT Imprint Code 44;344 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-434-11 1 in 1 CARTON 04/14/1998 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M011 04/14/1998 Labeler - United Natural Foods, Inc. dba UNFI (943556183) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(41163-434) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(41163-434) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(41163-434) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(41163-434) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(41163-434)