Label: VENELEX-
- NHRIC Code(s): 58980-780-11, 58980-780-21, 58980-780-50
- Packager: STRATUS PHARMACEUTICALS INC
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated April 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- ACTION
-
INDICATIONS
VENELEX™ OINTMENT is a wound dressing for topical use in the management of chronic and acute wounds, and dermal ulcers including: pressure ulcers (Stage I-IV), venous stasis ulcers, diabetic ulcers, first and second degree burns, surgical wounds, traumatic wounds, superficial wounds, ulcers resulting from arterial insufficiency and grafted wound/donor sites.
- CONTRAINDICATIONS
- USES
- WARNING
- USUAL DOSAGE
-
HOW SUPPLIED
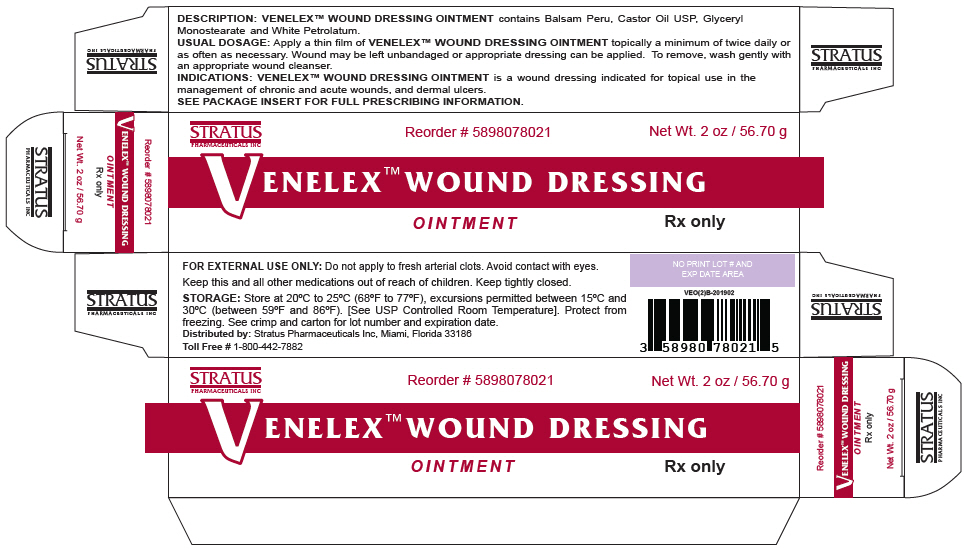
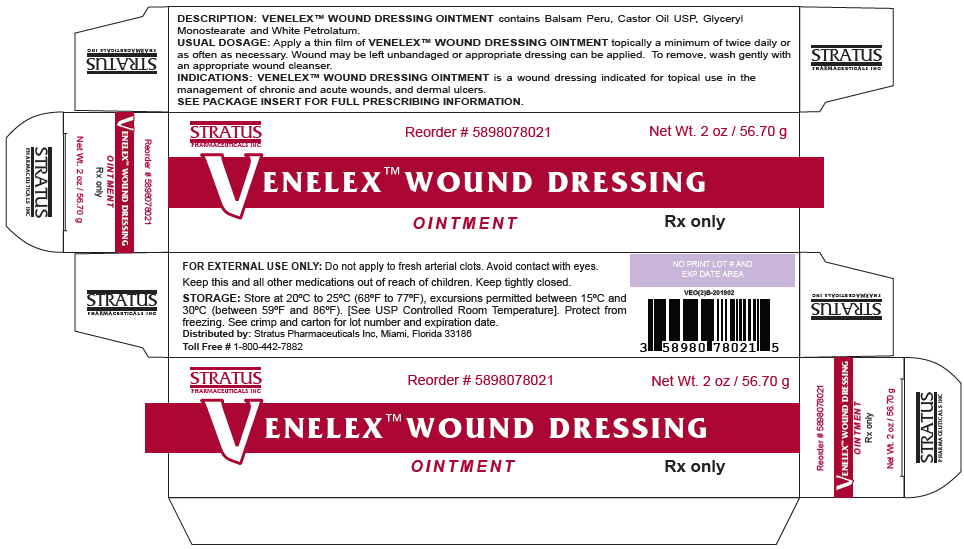
VENELEX™ OINTMENT is supplied as follows:
SIZE NDC NUMBER 30 GRAM TUBE 58980-780-11 60 GRAM TUBE 58980-780-21 5 GRAM PACKETTE × 60 58980-780-50 STORAGE
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized. [See USP Controlled Room Temperature]. Protect from freezing. See crimp for lot number and expiration date.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56.70 g Tube Box
-
INGREDIENTS AND APPEARANCE
VENELEX
dressing, wound, occlusiveProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:58980-780 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) BALSAM PERU (UNII: 8P5F881OCY) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:58980-780-11 1 in 1 BOX 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NHRIC:58980-780-21 1 in 1 BOX 2 60 g in 1 TUBE; Type 0: Not a Combination Product 3 NHRIC:58980-780-50 1 in 1 BOX, UNIT-DOSE 3 5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Exempt device NAD 09/15/2017 Labeler - STRATUS PHARMACEUTICALS INC (789001641) Establishment Name Address ID/FEI Business Operations TARMAC PRODUCTS INC 059890491 MANUFACTURE, LABEL, PACK