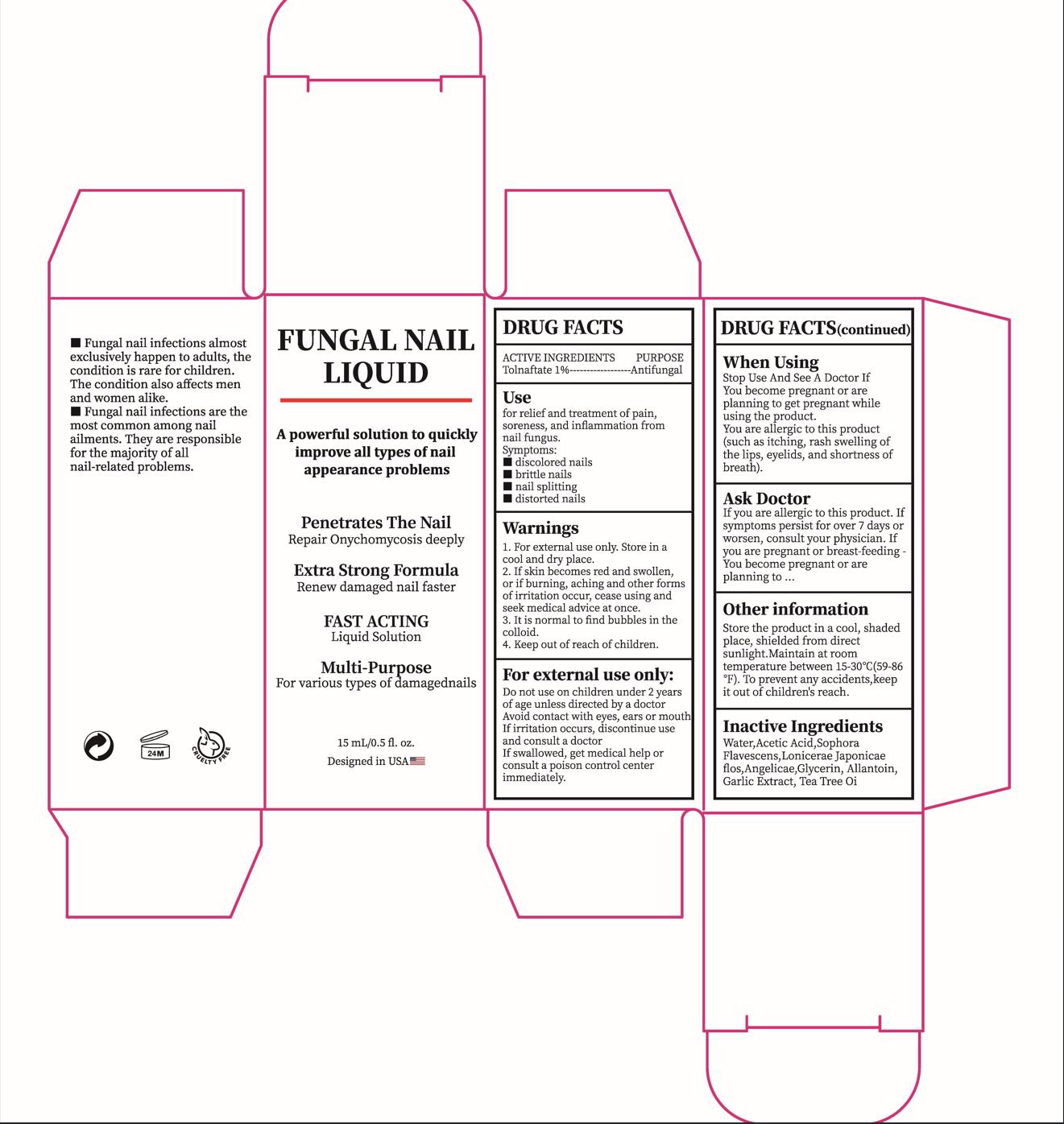
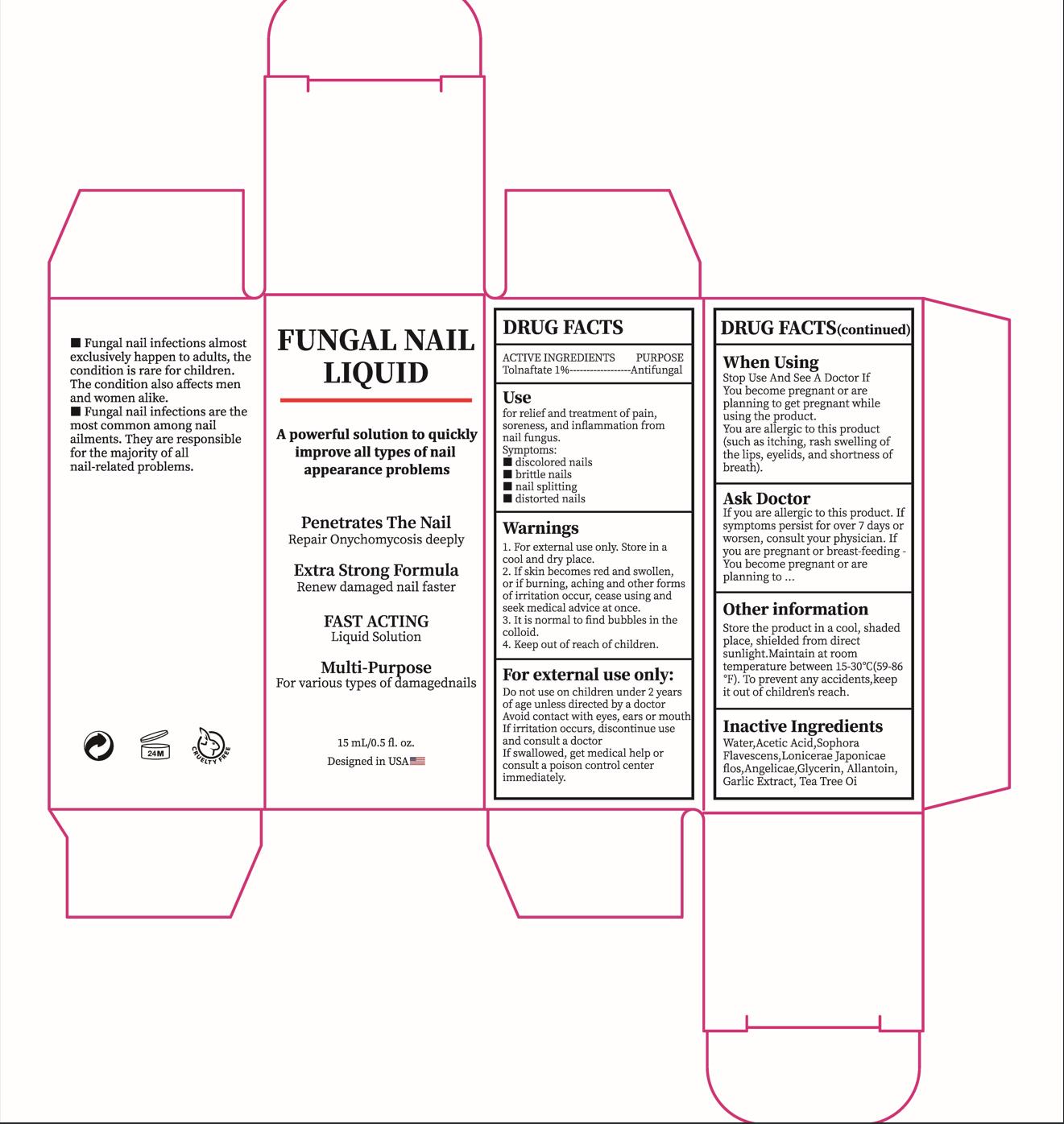
Label: FUNGAL NAIL LIQUID- tolnaftate liquid
- NDC Code(s): 84023-501-01
- Packager: Shenzhen Yangan Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
-
STOP USE
If skin becomes red and swollen, or if burning, aching and other forms ofirritation occur, cease using and seek medical advice at once.
If you are allergic to this product. Ifsymptoms persist for over 7 days orworsen, consult your physician.Ifyou are pregnant or breast-feeding You become pregnant or areplanning to
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGAL NAIL LIQUID
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84023-501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) GARLIC (UNII: V1V998DC17) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84023-501-01 1 in 1 BOX 03/08/2024 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 03/08/2024 Labeler - Shenzhen Yangan Technology Co., Ltd. (419283765) Establishment Name Address ID/FEI Business Operations Shenzhen Yangan Technology Co., Ltd. 419283765 manufacture(84023-501)