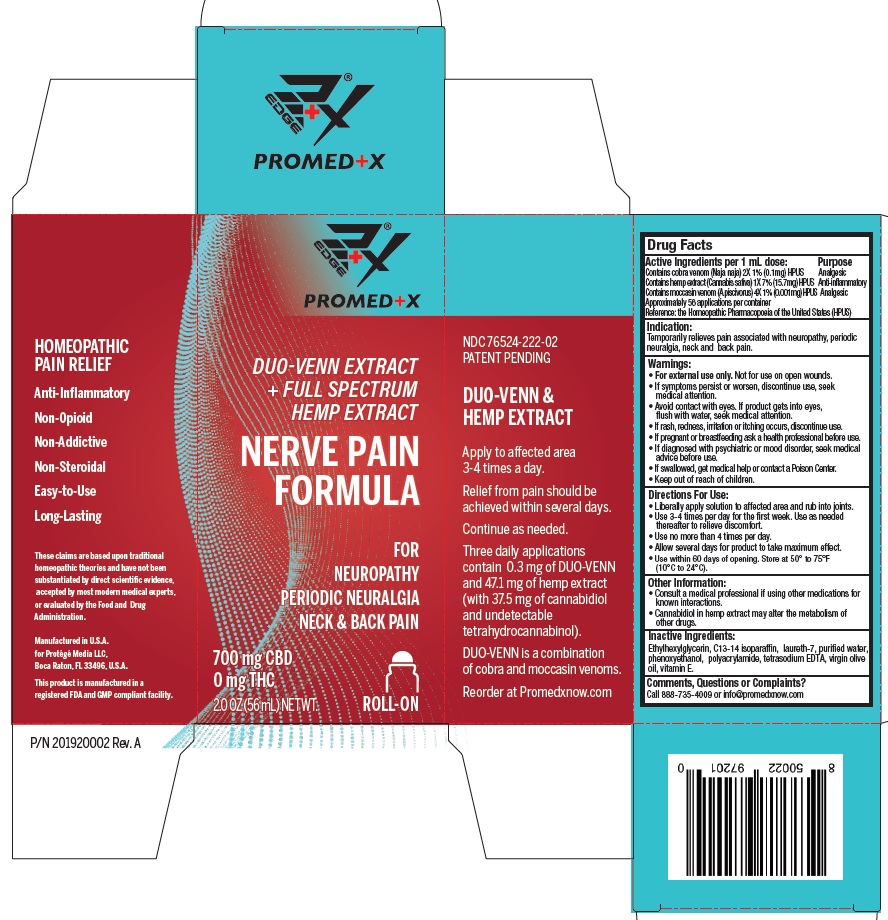
Label: NERVE PAIN FORMULA (cobra venom (naja naja) 2x, hemp extract (cannabis sativa) 1x, moccasin venom- a.piscivorus 4x lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 76524-222-02 - Packager: PROTEGE' MEDIA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 13, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients Purpose
- PURPOSE
- Indication:
- KEEP OUT OF REACH OF CHILDREN
-
Warnings:
• For external use only. Not for use on open wounds.
• If symptoms persist or worsen, discontinue use, seek medical attention.
• Avoid contact with eyes. If product gets into eyes, flush with water, seek medical attention.
• If rash, redness, irritation or itching occurs, discontinue use.
• If pregnant or breastfeeding ask a health professional before use.
• If diagnosed with psychiatric or mood disorder, seek medical advice before use.
• If swallowed, get medical help or contact a Poison Center.
• Keep out of reach of children. -
Directions For Use:
- Liberally apply lotion to affected area and rub into joints.
- Use 3-4 times per day for the first week. Use as needed thereafter to relieve discomfort.
- Use no more than 4 times per day.
- Allow several days for product to take maximum effect.
- Use within 60 days from opening. Store at 50° to 75°F (10°C to 24°C)
- Other Information:
- Inactive Ingredients:
- Comments, Questions or Complaints?
- PRODUCT LABEL
-
INGREDIENTS AND APPEARANCE
NERVE PAIN FORMULA
cobra venom (naja naja) 2x, hemp extract (cannabis sativa) 1x, moccasin venom (a.piscivorus) 4x lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76524-222 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 0.1 mg in 1 mL CANNABIS SATIVA SUBSP. SATIVA FLOWERING TOP (UNII: 8X454SZ22D) (CANNABIS SATIVA SUBSP. SATIVA FLOWERING TOP - UNII:8X454SZ22D) CANNABIS SATIVA SUBSP. SATIVA FLOWERING TOP 15.7 mg in 1 mL AGKISTRODON PISCIVORUS VENOM (UNII: X9V1Q8U150) (AGKISTRODON PISCIVORUS VENOM - UNII:X9V1Q8U150) AGKISTRODON PISCIVORUS VENOM 0.001 mg in 1 mL Inactive Ingredients Ingredient Name Strength C13-14 ISOPARAFFIN (UNII: E4F12ROE70) POLYACRYLAMIDE (CROSSLINKED; 0.01-0.2 MOLE PERCENT BISACRYLAMIDE) (UNII: RHA9LWJ494) LAURETH-7 (UNII: Z95S6G8201) WATER (UNII: 059QF0KO0R) OLIVE OIL (UNII: 6UYK2W1W1E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE SODIUM (UNII: MP1J8420LU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76524-222-02 56 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/29/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/29/2020 Labeler - PROTEGE' MEDIA LLC (117063923)