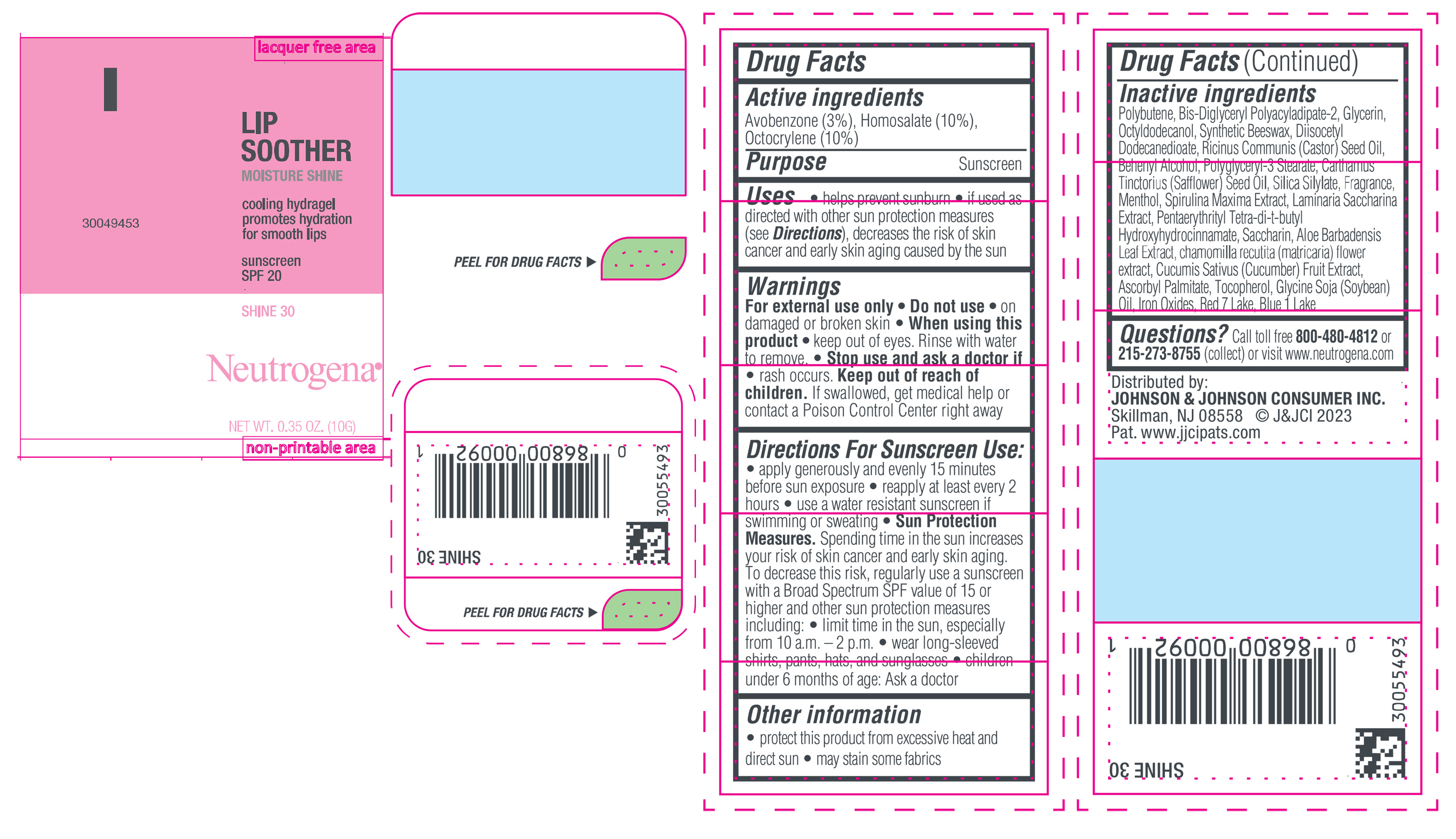
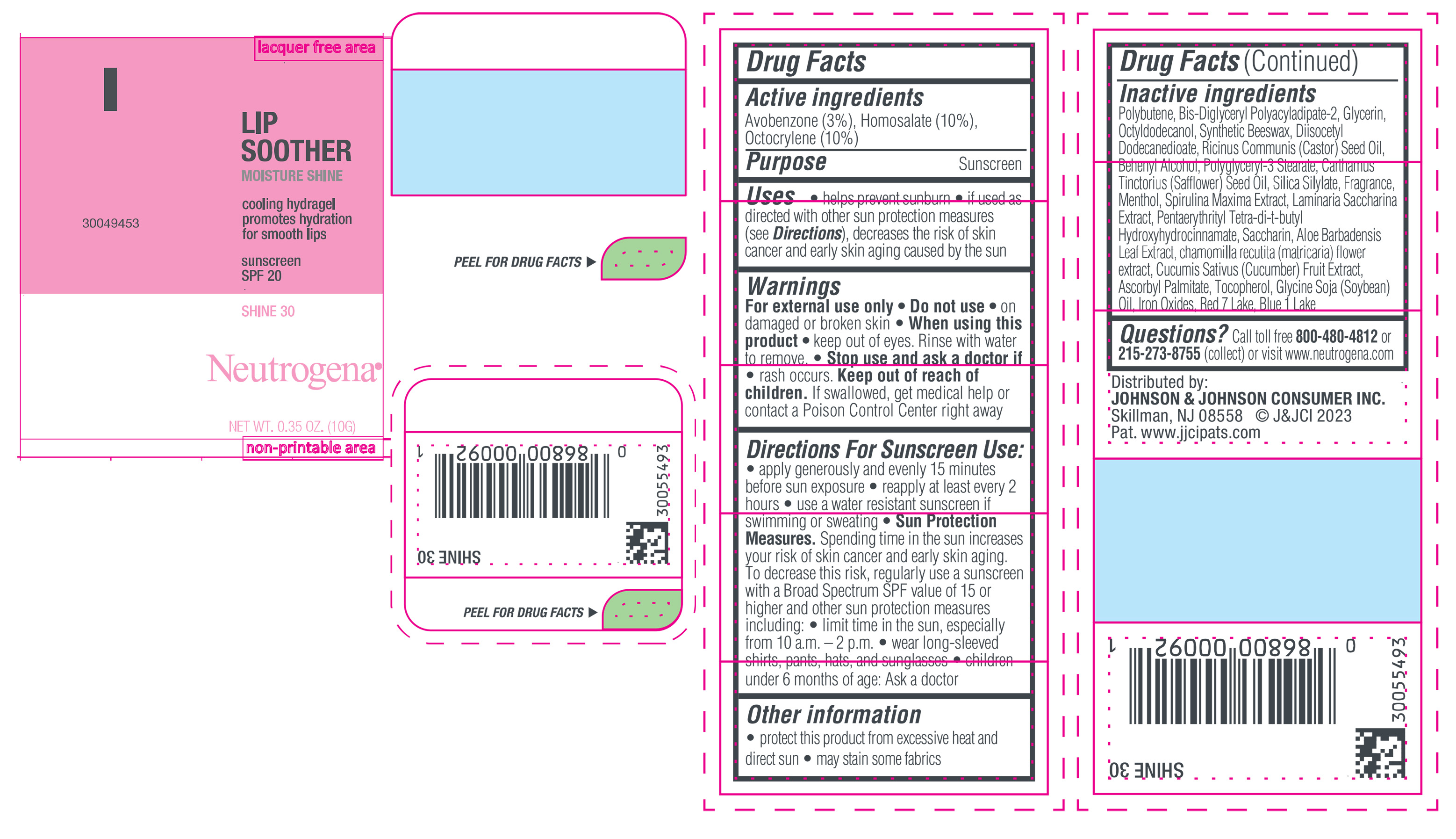
Label: NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, SHINE 30- avobenzone, homosalate, octocrylene gel
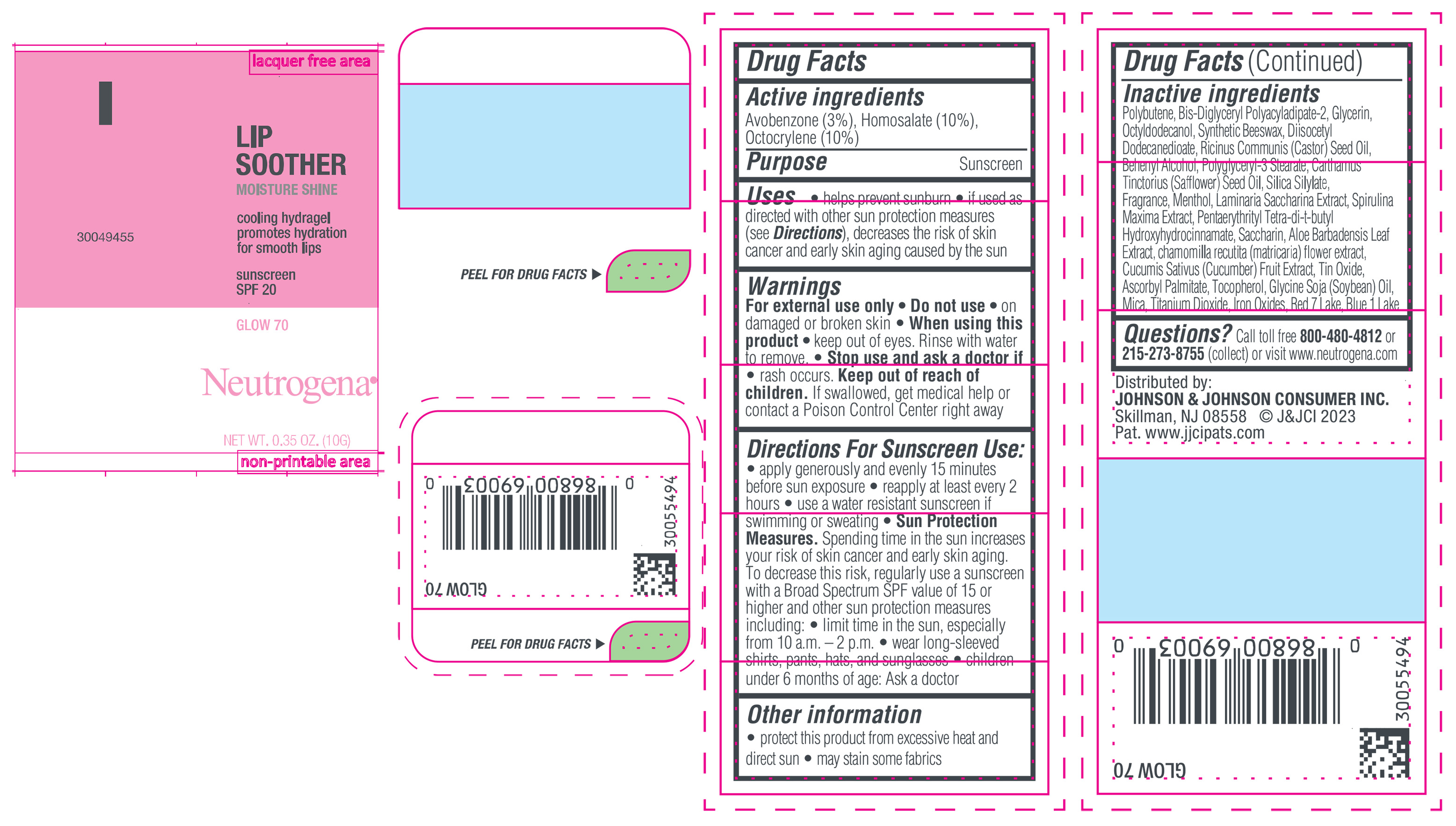
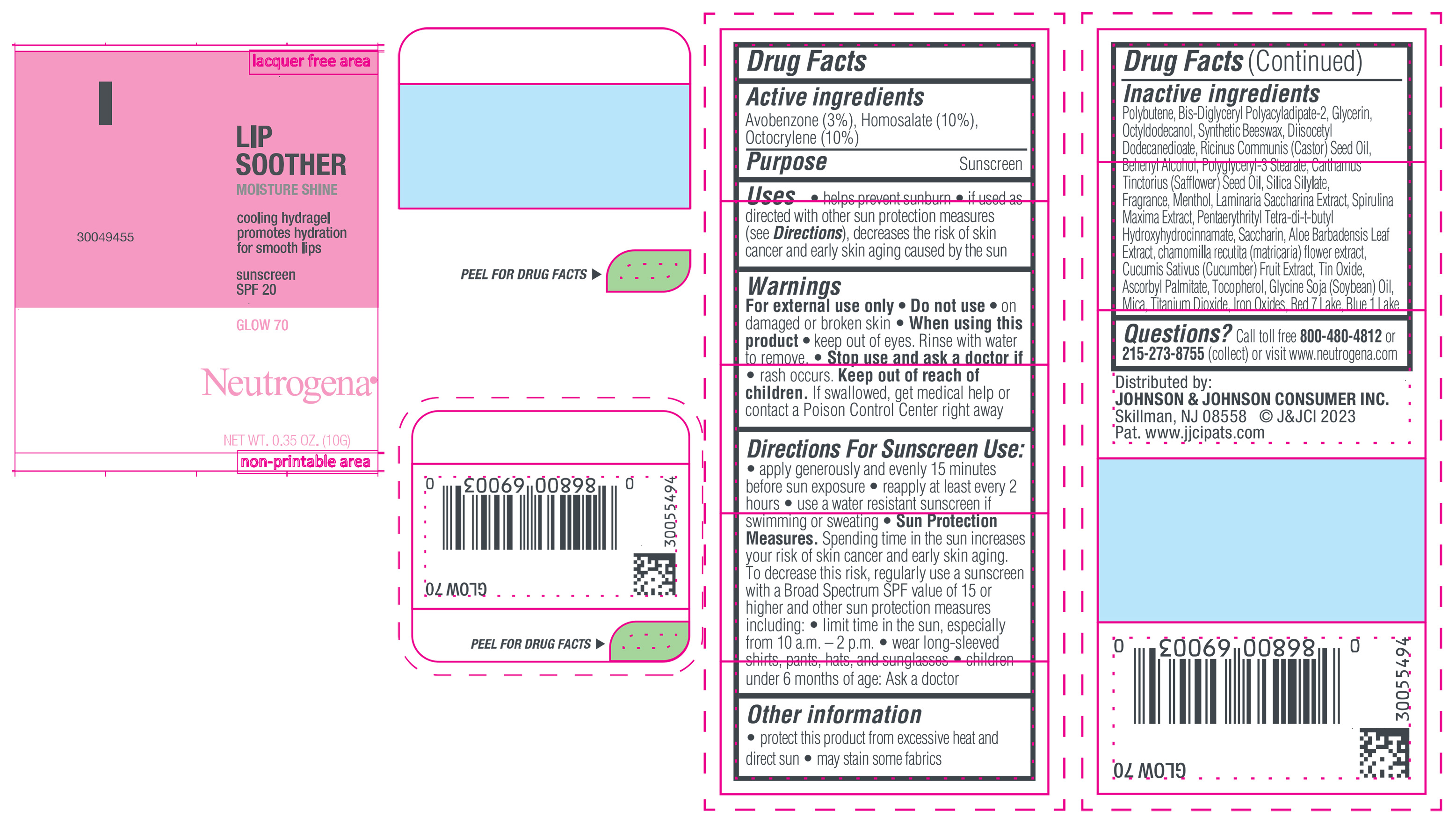
NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, GLOW 70- avobenzone, homosalate, octocrylene gel
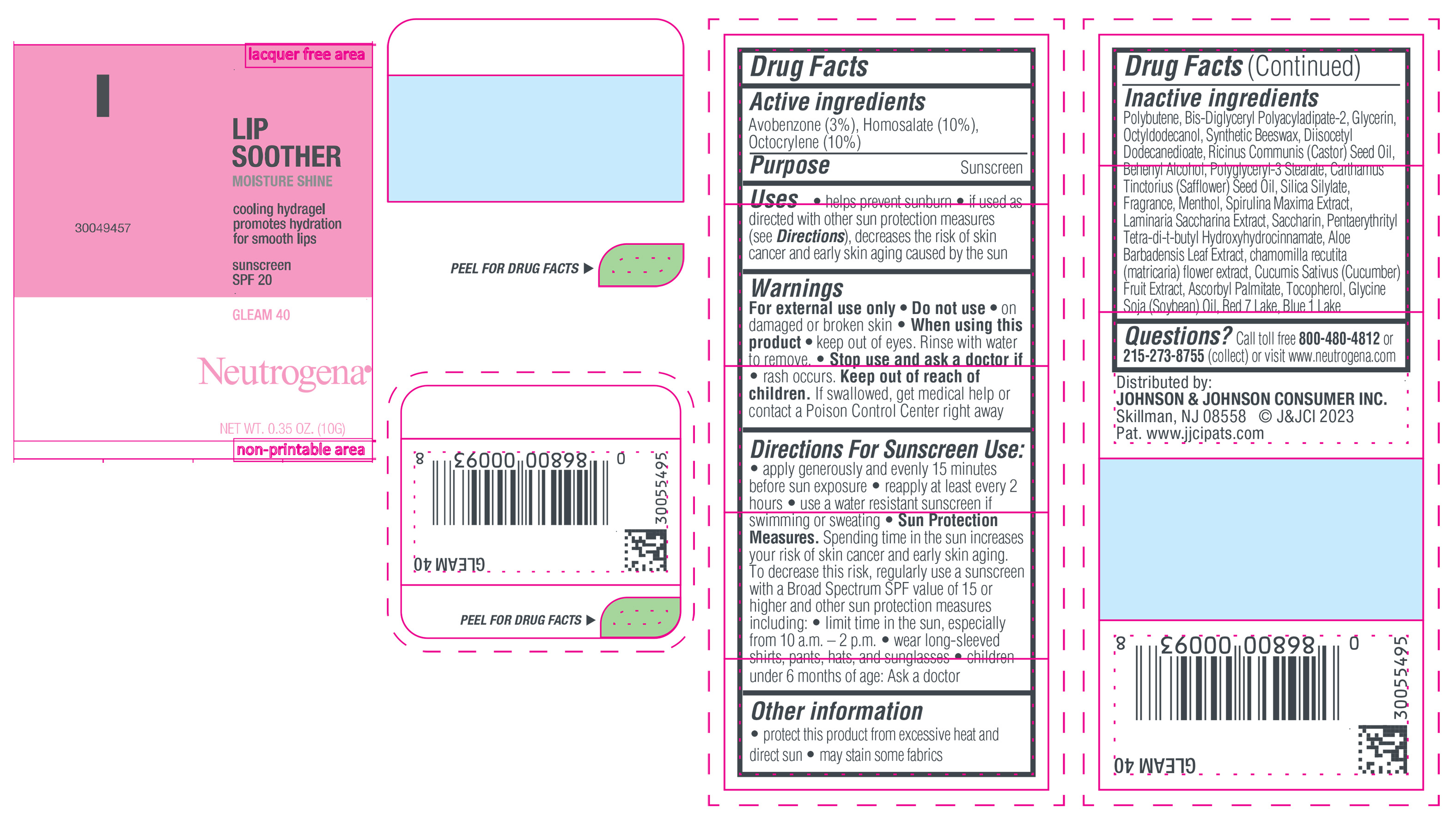
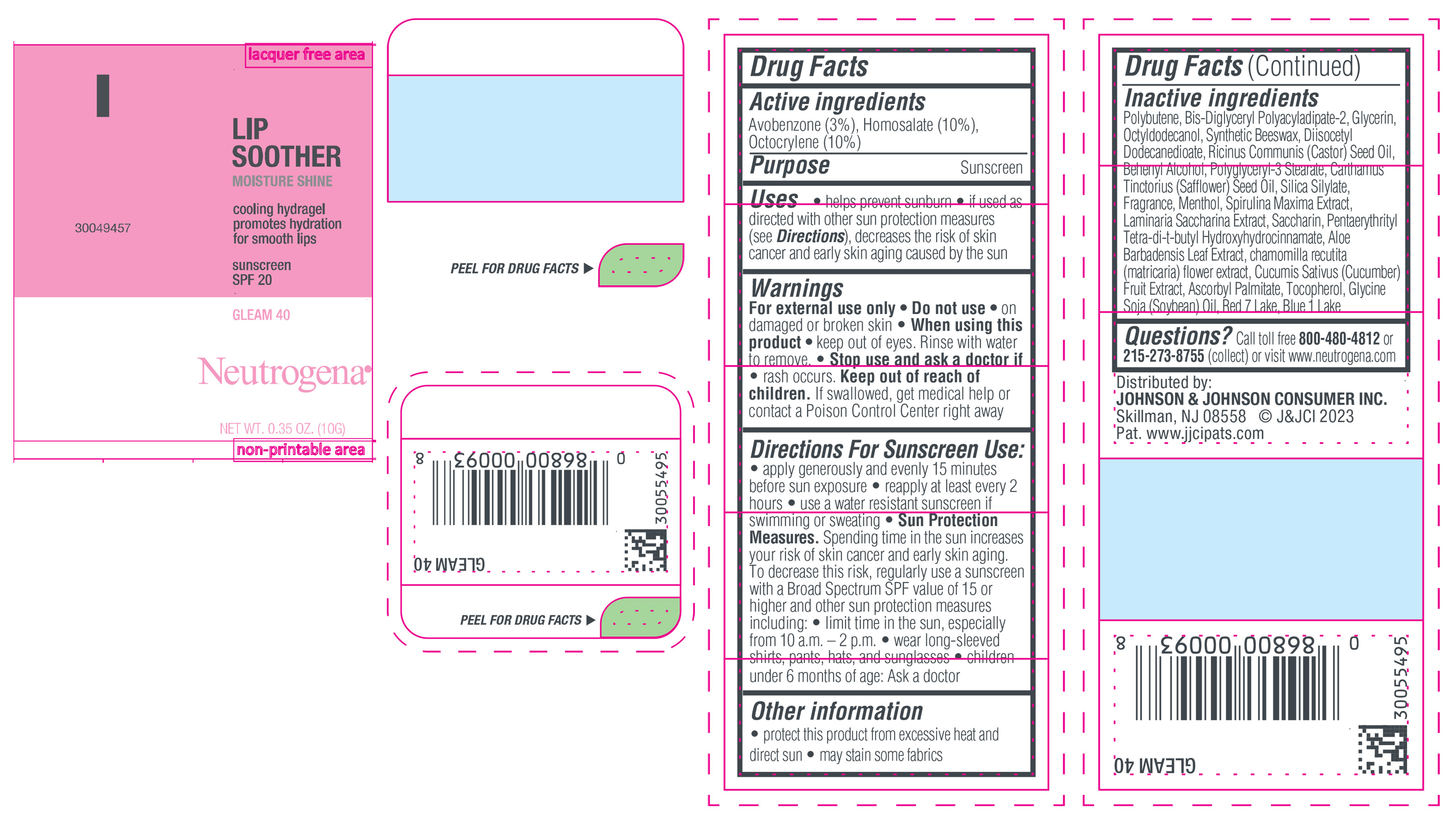
NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, GLEAM 40- avobenzone, homosalate, octocrylene gel
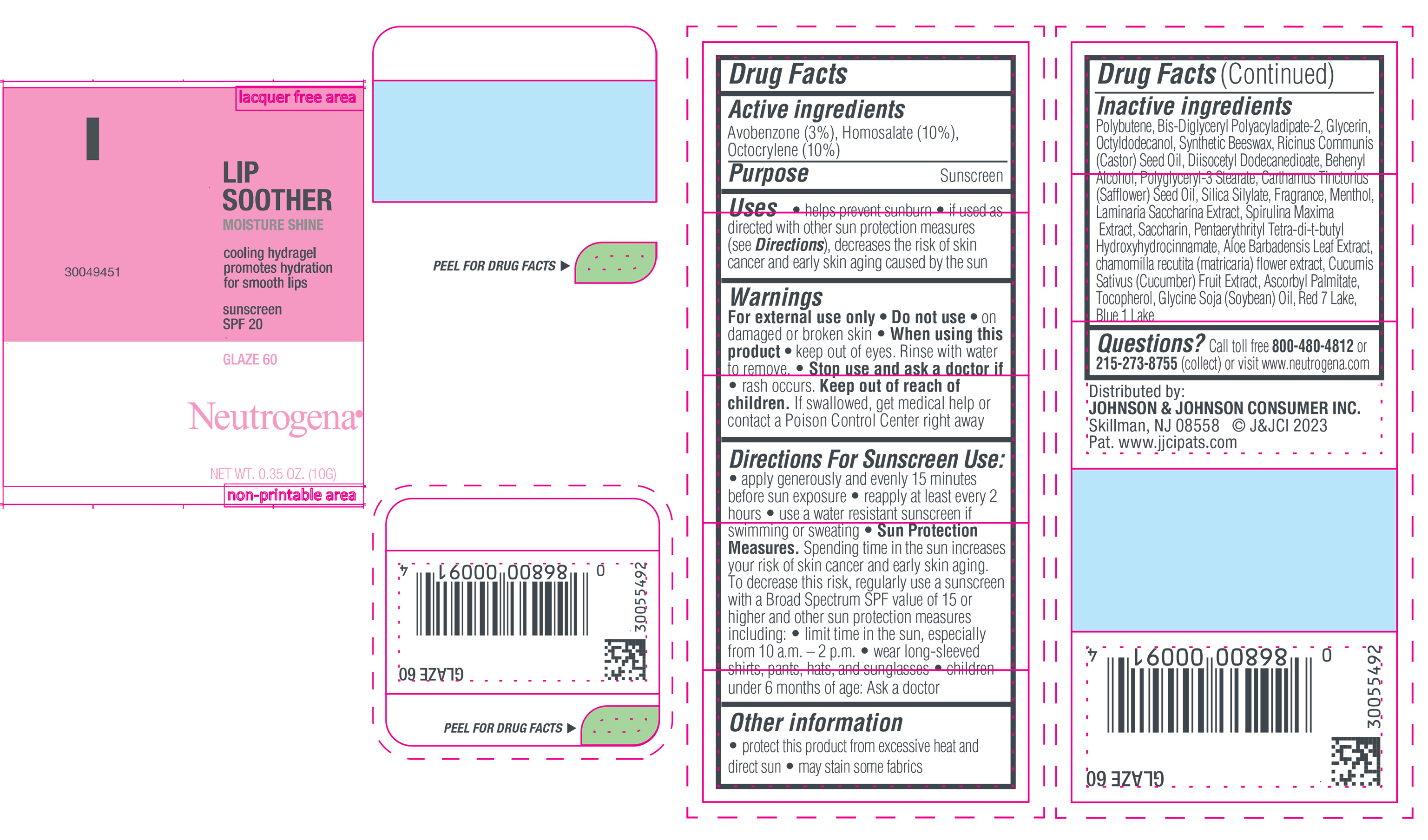
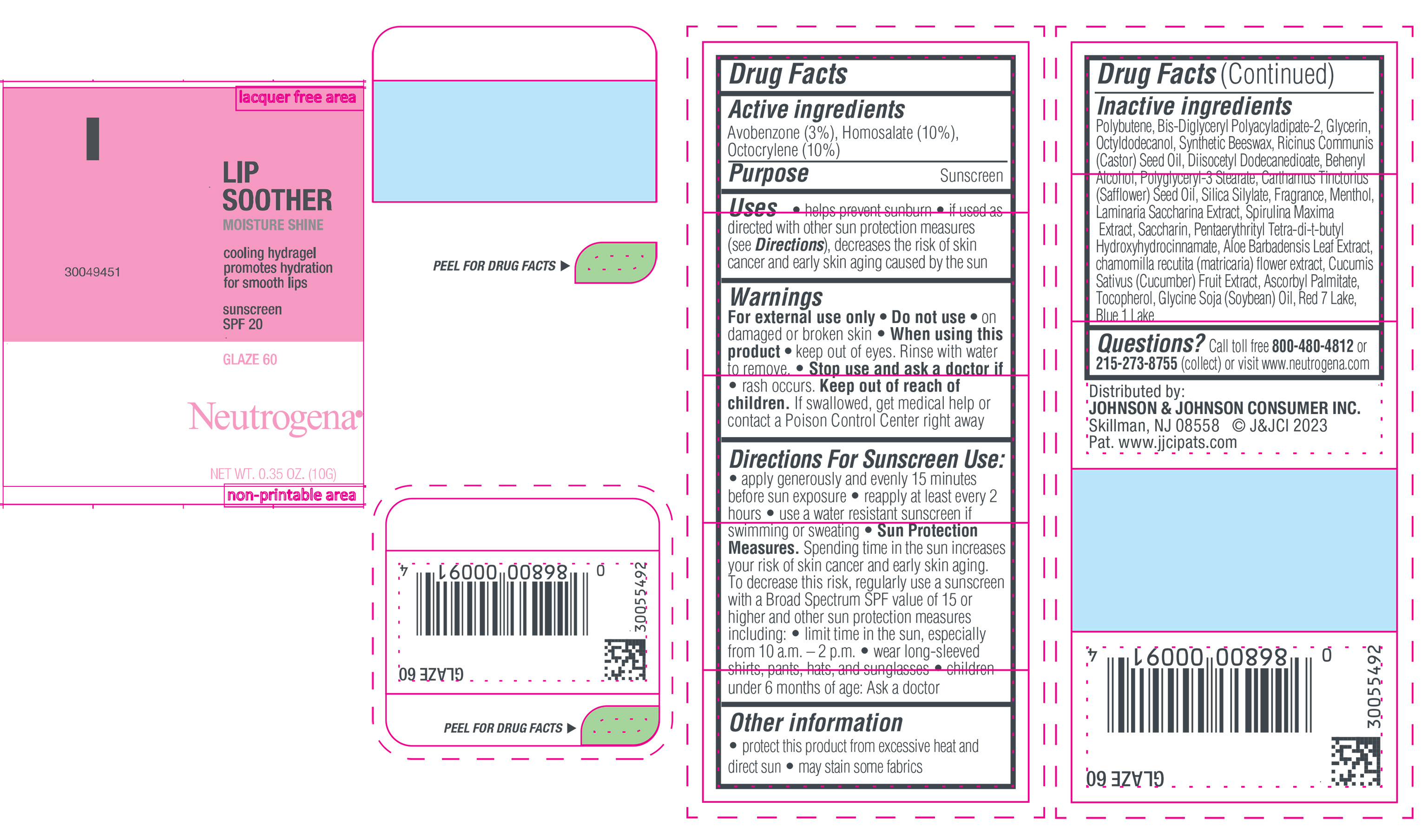
NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, GLAZE 60- avobenzone, homosalate, octocrylene gel
- NDC Code(s): 69968-0849-1, 69968-0850-1, 69968-0851-1, 69968-0852-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions For Sunscreen Use
• apply generously and evenly 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other Information
-
Inactive ingredients
Polybutene, Bis-Diglyceryl Polyacyladipate-2, Glycerin, Octyldodecanol, Synthetic Beeswax, Diisocetyl Dodecanedioate, Ricinus Communis (Castor) Seed Oil, Behenyl Alcohol, Polyglyceryl-3 Stearate, Carthamus Tinctorius (Safflower) Seed Oil, Silica Silylate, Fragrance, Menthol, Spirulina Maxima Extract, Laminaria Saccharina, Extract, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Saccharin, Aloe Barbadensis Leaf Extract, chamomilla recutita (matricaria) flower extract, Cucumis Sativus (Cucumber) Fruit Extract, Ascorbyl Palmitate, Tocopherol, Glycine Soja (Soybean) Oil, Iron Oxides, Red 7 Lake, Blue 1 Lake
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions For Sunscreen Use
• apply generously and evenly 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Polybutene, Bis-Diglyceryl Polyacyladipate-2, Glycerin, Octyldodecanol, Synthetic Beeswax, Diisocetyl Dodecanedioate, Ricinus Communis (Castor) Seed Oil, Behenyl Alcohol, Polyglyceryl-3 Stearate, Carthamus Tinctorius (Safflower) Seed Oil, Silica Silylate, Fragrance, Menthol, Spirulina Maxima Extract, Laminaria Saccharina, Extract, Saccharin, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Aloe Barbadensis Leaf Extract, chamomilla recutita (matricaria) flower extract, Cucumis Sativus (Cucumber) Fruit Extract, Ascorbyl Palmitate, Tocopherol, Glycine Soja (Soybean) Oil, Red 7 Lake, Blue 1 Lake
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions For Sunscreen Use
• apply generously and evenly 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Polybutene, Bis-Diglyceryl Polyacyladipate-2, Glycerin, Octyldodecanol, Synthetic Beeswax, Ricinus Communis (Castor) Seed Oil, Diisocetyl Dodecanedioate, , Behenyl Alcohol, Polyglyceryl-3 Stearate, Carthamus Tinctorius (Safflower) Seed Oil, Silica Silylate, Fragrance, Menthol, Laminaria Saccharina Extract, Spirulina Maxima Extract, , Saccharin , Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, , Aloe Barbadensis Leaf Extract, chamomilla recutita (matricaria) flower extract, Cucumis Sativus (Cucumber) Fruit Extract, Ascorbyl Palmitate, Tocopherol, Glycine Soja (Soybean) Oil, Red 7 Lake, Blue 1 Lake
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions For Sunscreen Use
• apply generously and evenly 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Polybutene, Bis-Diglyceryl Polyacyladipate-2, Glycerin, Octyldodecanol, Synthetic Beeswax, Diisocetyl Dodecanedioate, Ricinus Communis (Castor) Seed Oil, Behenyl Alcohol, Polyglyceryl-3 Stearate, Carthamus Tinctorius (Safflower) Seed Oil, Silica Silylate, Fragrance, Menthol, Laminaria Saccharina Extract, Spirulina Maxima Extract, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Saccharin, Aloe Barbadensis Leaf Extract, chamomilla recutita (matricaria) flower extract, Cucumis Sativus (Cucumber) Fruit Extract, Tin Oxide, Ascorbyl Palmitate, Tocopherol, Glycine Soja (Soybean) Oil, Mica, Titanium Dioxide, Iron Oxides, Red 7 Lake, Blue 1 Lake
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 g Tube Label, Shine 30
- PRINCIPAL DISPLAY PANEL - 10 g Tube Label, Gleam 40
- PRINCIPAL DISPLAY PANEL - 10 g Tube Label, Glaze 60
- PRINCIPAL DISPLAY PANEL - 10 g Tube Label, Glow 70
-
INGREDIENTS AND APPEARANCE
NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, SHINE 30
avobenzone, homosalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0849 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) DIISOCETYL DODECANEDIOATE (UNII: 1116DS0SNT) CASTOR OIL (UNII: D5340Y2I9G) POLYGLYCERYL-3 STEARATE (UNII: 8FDA8C98S3) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) OCTYLDODECANOL (UNII: 461N1O614Y) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) FERROUS OXIDE (UNII: G7036X8B5H) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) DOCOSANOL (UNII: 9G1OE216XY) SAFFLOWER OIL (UNII: 65UEH262IS) MENTHOL (UNII: L7T10EIP3A) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CUCUMBER (UNII: YY7C30VXJT) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) SPIRULINA MAXIMA (UNII: 9K7IG15M0Q) SACCHARIN (UNII: FST467XS7D) ASCORBYL PALMITATE (UNII: QN83US2B0N) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SOYBEAN OIL (UNII: 241ATL177A) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0849-1 10 g in 1 TUBE; Type 0: Not a Combination Product 04/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/11/2024 NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, GLOW 70
avobenzone, homosalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0852 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength STANNIC OXIDE (UNII: KM7N50LOS6) GLYCERIN (UNII: PDC6A3C0OX) DIISOCETYL DODECANEDIOATE (UNII: 1116DS0SNT) CASTOR OIL (UNII: D5340Y2I9G) POLYGLYCERYL-3 STEARATE (UNII: 8FDA8C98S3) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) OCTYLDODECANOL (UNII: 461N1O614Y) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) FERROUS OXIDE (UNII: G7036X8B5H) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) DOCOSANOL (UNII: 9G1OE216XY) SAFFLOWER OIL (UNII: 65UEH262IS) MENTHOL (UNII: L7T10EIP3A) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CUCUMBER (UNII: YY7C30VXJT) ASCORBYL PALMITATE (UNII: QN83US2B0N) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) SPIRULINA MAXIMA (UNII: 9K7IG15M0Q) SACCHARIN (UNII: FST467XS7D) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0852-1 10 g in 1 TUBE; Type 0: Not a Combination Product 04/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/11/2024 NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, GLEAM 40
avobenzone, homosalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) DIISOCETYL DODECANEDIOATE (UNII: 1116DS0SNT) CASTOR OIL (UNII: D5340Y2I9G) POLYGLYCERYL-3 STEARATE (UNII: 8FDA8C98S3) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) OCTYLDODECANOL (UNII: 461N1O614Y) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) DOCOSANOL (UNII: 9G1OE216XY) SAFFLOWER OIL (UNII: 65UEH262IS) MENTHOL (UNII: L7T10EIP3A) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CUCUMBER (UNII: YY7C30VXJT) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) SPIRULINA MAXIMA (UNII: 9K7IG15M0Q) SACCHARIN (UNII: FST467XS7D) ASCORBYL PALMITATE (UNII: QN83US2B0N) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SOYBEAN OIL (UNII: 241ATL177A) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0850-1 10 g in 1 TUBE; Type 0: Not a Combination Product 04/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/11/2024 NEUTROGENA MOISTURESHINE LIP SOOTHER WITH SUNSCREEN SPF 20, GLAZE 60
avobenzone, homosalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0851 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) DIISOCETYL DODECANEDIOATE (UNII: 1116DS0SNT) CASTOR OIL (UNII: D5340Y2I9G) POLYGLYCERYL-3 STEARATE (UNII: 8FDA8C98S3) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) OCTYLDODECANOL (UNII: 461N1O614Y) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) DOCOSANOL (UNII: 9G1OE216XY) SAFFLOWER OIL (UNII: 65UEH262IS) MENTHOL (UNII: L7T10EIP3A) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CUCUMBER (UNII: YY7C30VXJT) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) SPIRULINA MAXIMA (UNII: 9K7IG15M0Q) SACCHARIN (UNII: FST467XS7D) ASCORBYL PALMITATE (UNII: QN83US2B0N) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SOYBEAN OIL (UNII: 241ATL177A) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0851-1 10 g in 1 TUBE; Type 0: Not a Combination Product 04/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/11/2024 Labeler - Johnson & Johnson Consumer Inc. (118772437)