Label: BRUSH ON BLOCK BOUNCE BUTTER SPF 30 MOISTURIZING MOUSSE- zinc oxide lotion
- NDC Code(s): 58274-014-01
- Packager: SPF Ventures LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
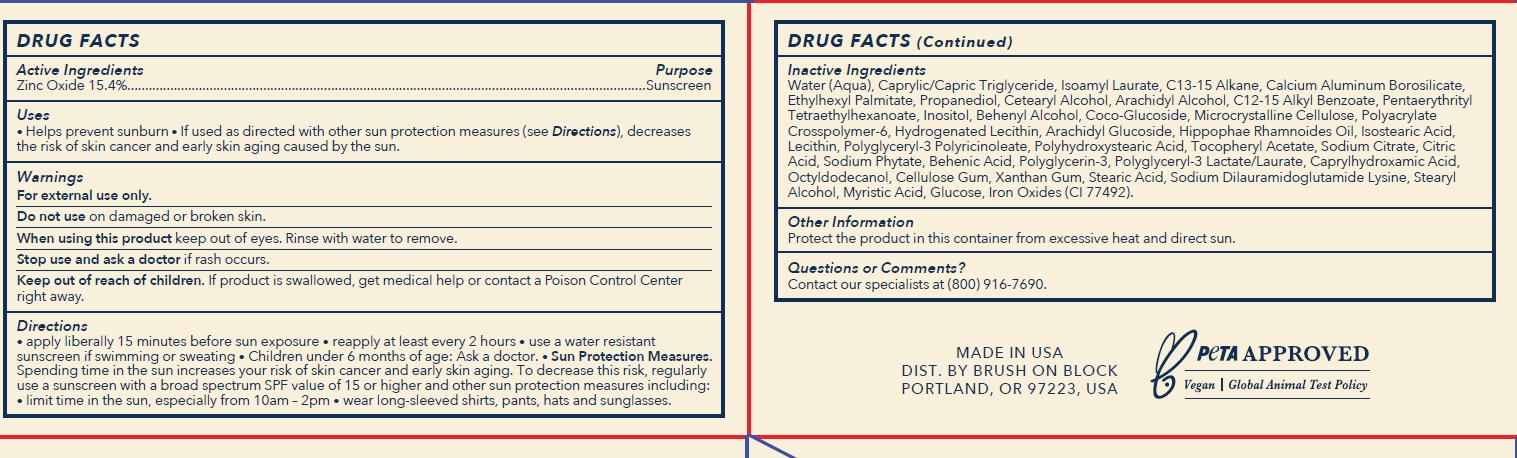
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure.
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor.
- Sunprotection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am-2pm
- wear long-sleeved shirts, pants, hats and sunglasses.
-
Inactive Ingredients
WATER (AQUA)
CAPRYLIC/CAPRIC TRIGLYCERIDE
ISOAMYL LAURATE
C13-15 ALKANE
CALCIUM SODIUM BOROSILICATE
ETHYLHEXYL PALMITATE
PROPANEDIOL
CETEARYL ALCOHOL
ARACHIDYL ALCOHOL
C12-15 ALKYL BENZOATE
PENTAERYTHRITYL TETRAETHYLHEXANOATE
ARACHIDYL GLUCOSIDE
BEHENIC ACID
BEHENYL ALCOHOL
CAPRYLHYDROXAMIC ACID
CELLULOSE GUM
CITRIC ACID
COCO-GLUCOSIDE
GLUCOSE
HIPPOPHAE RHAMNOIDES OIL
HYDROGENATED LECITHIN
INOSITOL
ISOSTEARIC ACID
LECITHIN
MICROCRYSTALLINE CELLULOSE
MYRISTIC ACID
OCTYLDODECANOL
POLYACRYLATE CROSSPOLYMER-6
POLYGLYCERIN-3
POLYGLYCERYL-3 LACTATE/LAURATE
POLYGLYCERYL-3 POLYRICINOLEATE
POLYHYDROXYSTEARIC ACID
SODIUM CITRATE
SODIUM DILAURAMIDOGLUTAMIDE LYSINE
SODIUM PHYTATE
STEARIC ACID
STEARYL ALCOHOL
T-BUTYL ALCOHOL
TOCOPHERYL ACETATE
XANTHAN GUM
IRON OXIDE (YELLOW) CI 77492
- Other Information
- label
-
INGREDIENTS AND APPEARANCE
BRUSH ON BLOCK BOUNCE BUTTER SPF 30 MOISTURIZING MOUSSE
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58274-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 15.4 g in 100 mL Inactive Ingredients Ingredient Name Strength STEARYL ALCOHOL (UNII: 2KR89I4H1Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) PHYTATE SODIUM (UNII: 88496G1ERL) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) ETHYLHEXYL PALMITATE (UNII: 2865993309) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) BEHENIC ACID (UNII: H390488X0A) DOCOSANOL (UNII: 9G1OE216XY) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) STEARIC ACID (UNII: 4ELV7Z65AP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) OCTYLDODECANOL (UNII: 461N1O614Y) PROPANEDIOL (UNII: 5965N8W85T) C13-15 ALKANE (UNII: 114P5I43UJ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PENTAERYTHRITYL TETRAETHYLHEXANOATE (UNII: XJ7052W897) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) COCO GLUCOSIDE (UNII: ICS790225B) HIPPOPHAE RHAMNOIDES FRUIT OIL (UNII: TA4JCF9S1J) INOSITOL (UNII: 4L6452S749) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) WATER (UNII: 059QF0KO0R) ISOAMYL LAURATE (UNII: M1SLX00M3M) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) POLYGLYCERYL-3 LAURATE (UNII: Y9ZSR39D0E) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) ISOSTEARIC ACID (UNII: X33R8U0062) MYRISTIC ACID (UNII: 0I3V7S25AW) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58274-014-01 100 mL in 1 JAR; Type 0: Not a Combination Product 03/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2024 Labeler - SPF Ventures LLC (055483891) Establishment Name Address ID/FEI Business Operations Innovation Labs, Inc 117109069 manufacture(58274-014)