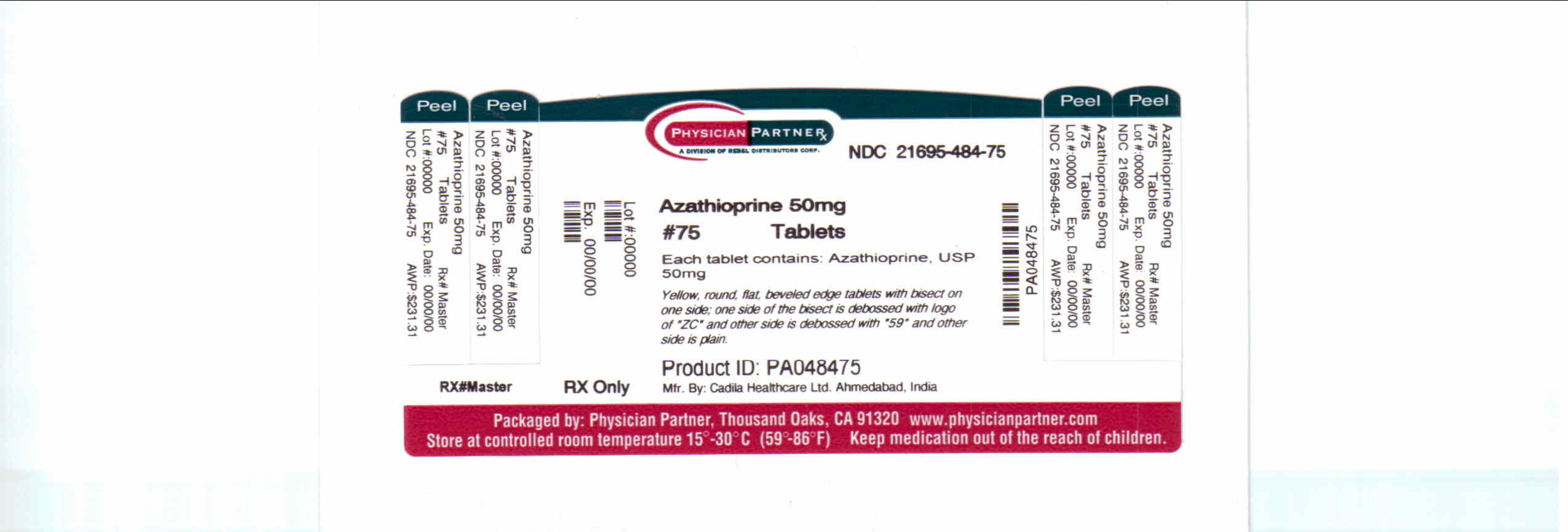
Label: AZATHIOPRINE- azathioprine tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-484-75 - Packager: Rebel Distributors Corp..
- This is a repackaged label.
- Source NDC Code(s): 65841-602
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 24, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: Chronic immunosuppression with this purine antimetabolite increases risk of neoplasia in humans. Physicians using this drug should be very familiar with this risk as well as with the mutagenic potential to both men and women and with possible hematologic toxicities (see WARNINGS).
-
DESCRIPTION
Azathioprine is an immunosuppressive antimetabolite. Each uncoated azathioprine tablet intended for oral administration contains 50 mg of azathioprine. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone and starch.
Azathioprine is chemically 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-1H-purine. The structural formula of azathioprine is:
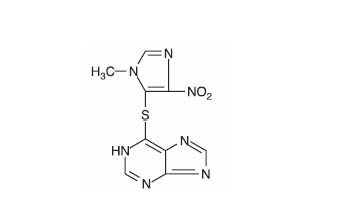
It is an imidazolyl derivative of 6-mercaptopurine and many of its biological effects are similar to those of the parent compound.
Azathioprine is a pale yellow, odorless powder. It is insoluble in water, soluble in dilute solutions of alkali hydroxides, sparingly soluble in dilute mineral acids, very slightly soluble in alcohol and in chloroform. The sodium salt of azathioprine is sufficiently soluble to make a 10 mg/mL water solution which is stable for 24 hours at 59° to 77°F (15° to 25°C). Azathioprine is stable in solution at neutral or acid pH but hydrolysis to mercaptopurine occurs in excess sodium hydroxide (0.1N), especially on warming. Conversion to mercaptopurine also occurs in the presence of sulfhydryl compounds such as cysteine, glutathione, and hydrogen sulfide.
-
CLINICAL PHARMACOLOGY
Azathioprine is well absorbed following oral administration. Maximum serum radioactivity occurs at 1 to 2 hours after oral 35S-azathioprine and decays with a half-life of 5 hours. This is not an estimate of the half-life of azathioprine itself, but is the decay rate for all 35S-containing metabolites of the drug. Because of extensive metabolism, only a fraction of the radioactivity is present as azathioprine. Usual doses produce blood levels of azathioprine, and of mercaptopurine derived from it, which are low (<1 mcg/mL). Blood levels are of little predictive value for therapy since the magnitude and duration of clinical effects correlate with thiopurine nucleotide levels in tissues rather than with plasma drug levels. Azathioprine and mercaptopurine are moderately bound to serum proteins (30%) and are partially dialyzable (see OVERDOSAGE).
Azathioprine is metabolized to 6-mercaptopurine (6-MP). Both compounds are rapidly eliminated from blood and are oxidized or methylated in erythrocytes and liver; no azathioprine or mercaptopurine is detectable in urine after 8 hours. Activation of 6-mercaptopurine occurs via hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and a series of multi-enzymatic processes involving kinases to form 6-thioguanine nucleotides (6-TGNs) as major metabolites (see Metabolism Scheme in Figure 1). The cytotoxicity of azathioprine is due, in part, to the incorporation of 6-TGN into DNA.
6-MP undergoes two major inactivation routes (Figure 1). One is thiol methylation, which is catalyzed by the enzyme thiopurine S-methyltransferase (TPMT), to form the inactive metabolite methyl-6-MP (6-MeMP). TPMT activity is controlled by a genetic polymorphism.1, 2, 3 For Caucasians and African Americans, approximately 10% of the population inherit one non-functional TPMT allele (heterozygous) conferring intermediate TPMT activity, and 0.3% inherit two TPMT non-functional alleles (homozygous) for low or absent TPMT activity. Non-functional alleles are less common in Asians. TPMT activity correlates inversely with 6-TGN levels in erythrocytes and presumably other hematopoietic tissues, since these cells have negligible xanthine oxidase (involved in the other inactivation pathway) activities, leaving TPMT methylation as the only inactivation pathway. Patients with intermediate TPMT activity may be at increased risk of myelotoxicity if receiving conventional doses of azathioprine tablets. Patients with low or absent TPMT activity are at an increased risk of developing severe, life-threatening myelotoxicity if receiving conventional doses of azathioprine tablets.4-9 TPMT genotyping or phenotyping (red blood cell TPMT activity) can help identify patients who are at an increased risk for developing azathioprine tablets toxicity.2, 3, 7, 8, 9 Accurate phenotyping (red blood cell TPMT activity) results are not possible in patients who have received recent blood transfusions (see WARNINGS, PRECAUTIONS: Drug Interactions, PRECAUTIONS: Laboratory Testsand ADVERSE REACTIONSsections).
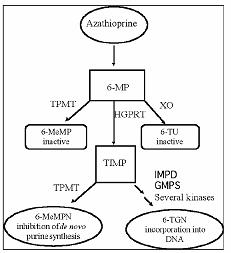
Figure 1. Metabolism pathway of azathioprine: competing pathways result in inactivation by TPMT or XO, or incorporation of cytotoxic nucleotides into DNA.
GMPS: Guanosine monophosphate synthetase; HGPRT: Hypoxanthine-guanine-phosphoribosyl-transferase; IMPD: Inosine monophosphate dehydrogenase; MeMP: Methylmercaptopurine; MeMPN: Methylmercaptopurine nucleotide; TGN: Thioguanine nucleotides; TIMP: Thioinosine monophosphate; TPMT: Thiopurine S-methyltransferase; TU: Thiouric acid; XO: Xanthine oxidase) (Adapted from Pharmacogenomics 2002; 3:89-98; and Cancer Res 2001; 61:5810-5816.)
Another inactivation pathway is oxidation, which is catalyzed by xanthine oxidase (XO) to form 6-thiouric acid. The inhibition of xanthine oxidase in patients receiving allopurinol (ZYLOPRIM®) is the basis for the azathioprine dosage reduction required in these patients (see PRECAUTIONS: Drug Interactions). Proportions of metabolites are different in individual patients, and this presumably accounts for variable magnitude and duration of drug effects. Renal clearance is probably not important in predicting biological effectiveness or toxicities, although dose reduction is practiced in patients with poor renal function.
Homograft Survival
The use of azathioprine for inhibition of renal homograft rejection is well established, the mechanism(s) for this action are somewhat obscure. The drug suppresses hypersensitivities of the cell-mediated type and causes variable alterations in antibody production. Suppression of T-cell effects, including ablation of T-cell suppression, is dependent on the temporal relationship to antigenic stimulus or engraftment. This agent has little effect on established graft rejections or secondary responses.
Alterations in specific immune responses or immunologic functions in transplant recipients are difficult to relate specifically to immunosuppression by azathioprine. These patients have subnormal responses to vaccines, low numbers of T-cells, and abnormal phagocytosis by peripheral blood cells, but their mitogenic responses, serum immunoglobulins, and secondary antibody responses are usually normal.
Immunoinflammatory Response
Azathioprine suppresses disease manifestations as well as underlying pathology in animal models of autoimmune disease. For example, the severity of adjuvant arthritis is reduced by azathioprine.
The mechanisms whereby azathioprine affects autoimmune diseases are not known. Azathioprine is immunosuppressive, delayed hypersensitivity and cellular cytotoxicity tests being suppressed to a greater degree than are antibody responses. In the rat model of adjuvant arthritis, azathioprine has been shown to inhibit the lymph node hyperplasia, which precedes the onset of the signs of the disease. Both the immunosuppressive and therapeutic effects in animal models are dose-related. Azathioprine is considered a slow-acting drug and effects may persist after the drug has been discontinued.
-
INDICATIONS AND USAGE
Azathioprine tablets are indicated as an adjunct for the prevention of rejection in renal homotransplantation. It is also indicated for the management of active rheumatoid arthritis to reduce signs and symptoms.
Renal Homotransplantation
Azathioprine tablets are indicated as an adjunct for the prevention of rejection in renal homotransplantation. Experience with over 16,000 transplants shows a 5-year patient survival of 35% to 55%, but this is dependent on donor, match for HLA antigens, anti-donor or anti-B-cell alloantigen antibody, and other variables. The effect of azathioprine tablets on these variables has not been tested in controlled trials.
Rheumatoid Arthritis
Azathioprine tablets are indicated for the treatment of active rheumatoid arthritis (RA) to reduce signs and symptoms. Aspirin, non-steroidal anti-inflammatory drugs and/or low dose glucocorticoids may be continued during treatment with azathioprine tablets. The combined use of azathioprine tablets with disease modifying anti-rheumatic drugs (DMARDs) has not been studied for either added benefit or unexpected adverse effects. The use of azathioprine tablets with these agents cannot be recommended.
-
CONTRAINDICATIONS
Azathioprine tablets should not be given to patients who have shown hypersensitivity to the drug. Azathioprine tablets should not be used for treating rheumatoid arthritis in pregnant women. Patients with rheumatoid arthritis previously treated with alkylating agents (cyclophosphamide, chlorambucil, melphalan, or others) may have a prohibitive risk of neoplasia if treated with azathioprine tablets.
-
WARNINGS
Severe leukopenia, thrombocytopenia, macrocytic anemia, and/or pancytopenia may occur in patients being treated with azathioprine tablets. Severe bone marrow suppression may also occur. Patients with intermediate thiopurine S-methyl transferase (TPMT) activity may be at an increased risk of myelotoxicity if receiving conventional doses of azathioprine tablets. Patients with low or absent TPMT activity are at an increased risk of developing severe, life-threatening myelotoxicity if receiving conventional doses of azathioprine tablets. TPMT genotyping or phenotyping can help identify patients who are at an increased risk for developing azathioprine tablets toxicity2-9 (see PRECAUTIONS: Laboratory Tests). Hematologic toxicities are dose-related and may be more severe in renal transplant patients whose homograft is undergoing rejection. It is suggested that patients on azathioprine tablets have complete blood counts, including platelet counts, weekly during the first month, twice monthly for the second and third months of treatment, then monthly or more frequently if dosage alterations or other therapy changes are necessary. Delayed hematologic suppression may occur. Prompt reduction in dosage or temporary withdrawal of the drug may be necessary if there is a rapid fall in or persistently low leukocyte count, or other evidence of bone marrow depression. Leukopenia does not correlate with therapeutic effect; therefore the dose should not be increased intentionally to lower the white blood cell count.
Serious infections are a constant hazard for patients receiving chronic immunosuppression, especially for homograft recipients. Fungal, viral, bacterial, and protozoal infections may be fatal and should be treated vigorously. Reduction of azathioprine dosage and/or use of other drugs should be considered.
Azathioprine tablets are mutagenic in animals and humans, carcinogenic in animals, and may increase the patient’s risk of neoplasia. Renal transplant patients are known to have an increased risk of malignancy, predominantly skin cancer and reticulum cell or lymphomatous tumors. The risk of post-transplant lymphomas may be increased in patients who receive aggressive treatment with immunosuppressive drugs. The degree of immunosuppression is determined, not only by the immunosuppressive regimen, but also by a number of other patient factors. The number of immunosuppressive agents may not necessarily increase the risk of post-transplant lymphomas. However, transplant patients who receive multiple immunosuppressive agents may be at risk for over-immunosuppression; therefore, immunosuppressive drug therapy should be maintained at the lowest effective levels. Information is available on the spontaneous neoplasia risk in rheumatoid arthritis, and on neoplasia following immunosuppressive therapy of other autoimmune diseases. It has not been possible to define the precise risk of neoplasia due to azathioprine tablets.The data suggest the risk may be elevated in patients with rheumatoid arthritis, though lower than for renal transplant patients. However, acute myelogenous leukemia as well as solid tumors have been reported in patients with rheumatoid arthritis who have received azathioprine. Data on neoplasia in patients receiving azathioprine tabletscan be found under ADVERSE REACTIONS.
Azathioprine tablets have been reported to cause temporary depression in spermatogenesis and reduction in sperm viability and sperm count in mice at doses 10 times the human therapeutic dose;10 a reduced percentage of fertile matings occurred when animals received 5 mg/kg.11
Pregnancy
Pregnancy Category D.
Azathioprine tablets can cause fetal harm when administered to a pregnant woman. Azathioprine tablets should not be given during pregnancy without careful weighing of risk versus benefit. Whenever possible, use of azathioprine tablets in pregnant patients should be avoided. This drug should not be used for treating rheumatoid arthritis in pregnant women.12
Azathioprine tablets are teratogenic in rabbits and mice when given in doses equivalent to the human dose (5 mg/kg daily). Abnormalities included skeletal malformations and visceral anomalies.11
Limited immunologic and other abnormalities have occurred in a few infants born of renal allograft recipients on azathioprine tablets. In a detailed case report,13 documented lymphopenia, diminished IgG and IgM levels, CMV infection, and a decreased thymic shadow were noted in an infant born to a mother receiving 150 mg azathioprine and 30 mg prednisone daily throughout pregnancy. At 10 weeks most features were normalized. DeWitte et al reported pancytopenia and severe immune deficiency in a preterm infant whose mother received 125 mg azathioprine and 12.5 mg prednisone daily.14 There have been two published reports of abnormal physical findings. Williamson and Karp described an infant born with preaxial polydactyly whose mother received azathioprine 200 mg daily and prednisone 20 mg every other day during pregnancy.15 Tallent et al described an infant with a large myelomeningocele in the upper lumbar region, bilateral dislocated hips, and bilateral talipes equinovarus. The father was on long-term azathioprine therapy.16
Benefit versus risk must be weighed carefully before use of azathioprine tablets in patients of reproductive potential. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing age should be advised to avoid becoming pregnant.
-
PRECAUTIONS
General
A gastrointestinal hypersensitivity reaction characterized by severe nausea and vomiting has been reported. These symptoms may also be accompanied by diarrhea, rash, fever, malaise, myalgias, elevations in liver enzymes, and occasionally, hypotension. Symptoms of gastrointestinal toxicity most often develop within the first several weeks of therapy with azathioprine tablets and are reversible upon discontinuation of the drug. The reaction can recur within hours after rechallenge with a single dose of azathioprine tablets.
Information for Patients
Patients being started on azathioprine tablets should be informed of the necessity of periodic blood counts while they are receiving the drug and should be encouraged to report any unusual bleeding or bruising to their physician. They should be informed of the danger of infection while receiving azathioprine tablets and asked to report signs and symptoms of infection to their physician. Careful dosage instructions should be given to the patient, especially when azathioprine tablets are being administered in the presence of impaired renal function or concomitantly with allopurinol (see Drug Interactionssubsection and DOSAGE AND ADMINISTRATION). Patients should be advised of the potential risks of the use of azathioprine tablets during pregnancy and during the nursing period. The increased risk of neoplasia following therapy with azathioprine tablets should be explained to the patient.
Laboratory Tests
Complete Blood Count (CBC) Monitoring
Patients on azathioprine tablets should have complete blood counts, including platelet counts, weekly during the first month, twice monthly for the second and third months of treatment, then monthly or more frequently if dosage alterations or other therapy changes are necessary.
TPMT Testing
It is recommended that consideration be given to either genotype or phenotype patients for TPMT. Phenotyping and genotyping methods are commercially available. The most common non-functional alleles associated with reduced levels of TPMT activity are TPMT*2, TPMT*3A and TPMT*3C. Patients with two non-functional alleles (homozygous) have low or absent TPMT activity and those with one non-functional allele (heterozygous) have intermediate activity. Accurate phenotyping (red blood cell TPMT activity) results are not possible in patients who have received recent blood transfusions. TPMT testing may also be considered in patients with abnormal CBC results that do not respond to dose reduction. Early drug discontinuation in these patients is advisable. TPMT TESTING CANNOT SUBSTITUTE FOR COMPLETE BLOOD COUNT (CBC) MONITORING IN PATIENTS RECEIVING AZATHIOPRINE TABLETS (see CLINICAL PHARMACOLOGY, WARNINGS, ADVERSE REACTIONSand DOSAGE AND ADMINISTRATIONsections).
Drug Interactions
Use with Allopurinol
One of the pathways for inactivation of azathioprine is inhibited by allopurinol. Patients receiving azathioprine tablets and allopurinol concomitantly should have a dose reduction of azathioprine tablets, to approximately ⅓ to ¼ the usual dose. It is recommended that a further dose reduction or alternative therapies be considered for patients with low or absent TPMT activity receiving azathioprine tablets and allopurinol because both TPMT and XO inactivation pathways are affected (see CLINICAL PHARMACOLOGY, WARNINGS, PRECAUTIONS: Laboratory Testsand ADVERSE REACTIONSsections).
Use with Aminosalicylates
There is in vitro evidence that aminosalicylate derivatives (e.g., sulphasalazine, mesalazine, or olsalazine) inhibit the TPMT enzyme. Concomitant use of these agents with azathioprine tablets should be done with caution.
Use with Other Agents Affecting Myelopoesis
Drugs which may affect leukocyte production, including co-trimoxazole, may lead to exaggerated leukopenia, especially in renal transplant recipients.
Use with Angiotensin-Converting Enzyme Inhibitors
The use of angiotensin-converting enzyme inhibitors to control hypertension in patients on azathioprine has been reported to induce anemia and severe leukopenia.
Use with Warfarin
Azathioprine tablets may inhibit the anticoagulant effect of warfarin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
See WARNINGSsection.
Nursing Mothers
The use of azathioprine tablets in nursing mothers is not recommended. Azathioprine or its metabolites are transferred at low levels, both transplacentally and in breast milk.17, 18, 19 Because of the potential for tumorigenicity shown for azathioprine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
The principal and potentially serious toxic effects of azathioprine tablets are hematologic and gastrointestinal. The risks of secondary infection and neoplasia are also significant (see WARNINGS). The frequency and severity of adverse reactions depend on the dose and duration of azathioprine tablets as well as on the patient’s underlying disease or concomitant therapies. The incidence of hematologic toxicities and neoplasia encountered in groups of renal homograft recipients is significantly higher than that in studies employing azathioprine tablets for rheumatoid arthritis. The relative incidences in clinical studies are summarized below:
Toxicity Renal Homograft Rheumatoid Arthritis Leukopenia (any degree) >50% 28% <2,500 cells/mm3 16% 5.3% Infections 20% <1% Neoplasia Lymphoma 0.5% * Others 2.8% Hematologic
Leukopenia and/or thrombocytopenia are dose-dependent and may occur late in the course of therapy with azathioprine tablets. Dose reduction or temporary withdrawal may result in reversal of these toxicities. Infection may occur as a secondary manifestation of bone marrow suppression or leukopenia, but the incidence of infection in renal homotransplantation is 30 to 60 times that in rheumatoid arthritis. Macrocytic anemia and/or bleeding have been reported.
TPMT genotyping or phenotyping can help identify patients with low or absent TPMT activity (homozygous for non-functional alleles) who are at increased risk for severe, life-threatening myelosuppression from azathioprine tablets (see CLINICAL PHARMACOLOGY, WARNINGSand PRECAUTIONS: Laboratory Tests). Death associated with pancytopenia has been reported in patients with absent TPMT activity receiving azathioprine.6, 20
Gastrointestinal
Nausea and vomiting may occur within the first few months of therapy with azathioprine tablets, and occurred in approximately 12% of 676 rheumatoid arthritis patients. The frequency of gastric disturbance often can be reduced by administration of the drug in divided doses and/or after meals. However, in some patients, nausea and vomiting may be severe and may be accompanied by symptoms such as diarrhea, fever, malaise, and myalgias (see PRECAUTIONS). Vomiting with abdominal pain may occur rarely with a hypersensitivity pancreatitis. Hepatotoxicity manifest by elevation of serum alkaline phosphatase, bilirubin, and/or serum transaminases is known to occur following azathioprine use, primarily in allograft recipients. Hepatotoxicity has been uncommon (less than 1%) in rheumatoid arthritis patients. Hepatotoxicity following transplantation most often occurs within 6 months of transplantation and is generally reversible after interruption of azathioprine tablets. A rare, but life-threatening hepatic veno-occlusive disease associated with chronic administration of azathioprine has been described in transplant patients and in one patient receiving azathioprine tablets for panuveitis.21, 22, 23 Periodic measurement of serum transaminases, alkaline phosphatase, and bilirubin is indicated for early detection of hepatotoxicity. If hepatic veno-occlusive disease is clinically suspected, azathioprine tablets should be permanently withdrawn.
-
OVERDOSAGE
The oral LD50s for single doses of azathioprine tablets in mice and rats are 2500 mg/kg and 400 mg/kg, respectively. Very large doses of this antimetabolite may lead to marrow hypoplasia, bleeding, infection, and death. About 30% of azathioprine is bound to serum proteins, but approximately 45% is removed during an 8-hour hemodialysis.24 A single case has been reported of a renal transplant patient who ingested a single dose of 7500 mg azathioprine. The immediate toxic reactions were nausea, vomiting, and diarrhea, followed by mild leukopenia and mild abnormalities in liver function. The white blood cell count, SGOT, and bilirubin returned to normal 6 days after the overdose.
-
DOSAGE AND ADMINISTRATION
TPMT TESTING CANNOT SUBSTITUTE FOR COMPLETE BLOOD COUNT (CBC) MONITORING IN PATIENTS RECEIVING AZATHIOPRINE TABLETS. TPMT genotyping or phenotyping can be used to identify patients with absent or reduced TPMT activity. Patients with low or absent TPMT activity are at an increased risk of developing severe, life-threatening myelotoxicity from azathioprine tablets if conventional doses are given. Physicians may consider alternative therapies for patients who have low or absent TPMT activity (homozygous for non-functional alleles). Azathioprine tablets should be administered with caution to patients having one non-functional allele (heterozygous) who are at risk for reduced TPMT activity that may lead to toxicity if conventional doses are given. Dosage reduction is recommended in patients with reduced TPMT activity. Early drug discontinuation may be considered in patients with abnormal CBC results that do not respond to dose reduction.
Renal Homotransplantation
The dose of azathioprine tablets required to prevent rejection and minimize toxicity will vary with individual patients; this necessitates careful management. The initial dose is usually 3 to 5 mg/kg daily, beginning at the time of transplant. Azathioprine tablets are usually given as a single daily dose on the day of, and in a minority of cases 1 to 3 days before, transplantation. Azathioprine is often initiated with the intravenous administration of the sodium salt, with subsequent use of tablets (at the same dose level) after the postoperative period. Intravenous administration of the sodium salt is indicated only in patients unable to tolerate oral medications. Dose reduction to maintenance levels of 1 to 3 mg/kg daily is usually possible. The dose of azathioprine tablets should not be increased to toxic levels because of threatened rejection. Discontinuation may be necessary for severe hematologic or other toxicity, even if rejection of the homograft may be a consequence of drug withdrawal.
Rheumatoid Arthritis
Azathioprine tablets are usually given on a daily basis. The initial dose should be approximately 1.0 mg/kg (50 to 100 mg) given as a single dose or on a twice-daily schedule. The dose may be increased, beginning at 6 to 8 weeks and thereafter by steps at 4-week intervals, if there are no serious toxicities and if initial response is unsatisfactory. Dose increments should be 0.5 mg/kg daily, up to a maximum dose of 2.5 mg/kg per day. Therapeutic response occurs after several weeks of treatment, usually 6 to 8; an adequate trial should be a minimum of 12 weeks. Patients not improved after 12 weeks can be considered refractory. Azathioprine tablets may be continued long-term in patients with clinical response, but patients should be monitored carefully, and gradual dosage reduction should be attempted to reduce risk of toxicities.
Maintenance therapy should be at the lowest effective dose, and the dose given can be lowered decrementally with changes of 0.5 mg/kg or approximately 25 mg daily every 4 weeks while other therapy is kept constant. The optimum duration of maintenance azathioprine tablets has not been determined. Azathioprine tablets can be discontinued abruptly, but delayed effects are possible.
- HOW SUPPLIED
-
REFERENCES
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43:329-339.
- Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine methyltransferase. Drug Metab Dispos. 2001;29:601-605.
- McLeod HL, Siva C. The thiopurine S-methyltransferase gene locus implications for clinical pharmacogenomics. Pharmacogenomics. 2002;3:89-98.
- Anstey A, Lennard L, Mayou SC, et al. Pancytopenia related to azathioprine – an enzyme deficiency caused by a common genetic polymorphism: a review. JR Soc Med. 1992; 85:752-756.
- Stolk JN, Beorbooms AM, de Abreu RA, et al. Reduced thiopurine methyltransferase activity and development of side effects of azathioprine treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1998; 41:1858-1866.
- Data on file, Prometheus Laboratories Inc.
- Yates CR, Krynetski EY, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997; 126:608-614.
- Black AJ, McLeod HL, Capell HA, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med. 1998; 129:716-718.
- Clunie GP, Lennard L. Relevance of thiopurine methyltransferase status in rheumatology patients receiving azathioprine. Rheumatology. 2004; 43:13-18.
- Clark JM. The mutagenicity of azathioprine in mice, Drosophila melanogaster, and Neurospora crassa. Mutat Res. 1975; 28:87-99.
- Data on file, Prometheus Laboratories Inc.
- Tagatz GE, Simmons RL. Pregnancy after renal transplantation. Ann Intern Med. 1975; 82:113-114. Editorial Notes.
- Cote’ CJ, Meuwissen HJ, Pickering RJ. Effects on the neonate of prednisone and azathioprine administered to the mother during pregnancy. J Pediatr. 1974; 85:324-328.
- DeWitte DB, Buick MK, Cyran SE, et al. Neonatal pancytopenia and severe combined immunodeficiency associated with antenatal administration of azathioprine and prednisone. J Pediatr. 1984; 105:625-628.
- Williamson RA, Karp LE. Azathioprine teratogenicity: review of the literature and case report. Obstet Gynecol. 1981; 58:247-250.
- Tallent MB, Simmons RL, Najarian JS. Birth defects in child of male recipient of kidney transplant. JAMA. 1970; 211: 1854-1855.
- Data on file, Prometheus Laboratories Inc.
- Saarikoski S, Seppälä M. Immunosuppression during pregnancy: transmission of azathioprine and its metabolites from the mother to the fetus. Am J Obstet Gynecol. 1973; 115:1100-1106.
- Coulam CB, Moyer TP, Jiang NS, et al. Breast-feeding after renal transplantation. Transplant Proc. 1982; 14: 605-609.
- Schutz E, Gummert J, Mohr F, Oellerich M. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant patients. Lancet. 1993; 341:436.
- Read AE, Wiesner RH, LaBrecque DR, et al. Hepatic veno-occlusive disease associated with renal transplantation and azathioprine therapy. Ann Intern Med. 1986; 104:651-655.
- Katzka DA, Saul SH, Jorkasky D, et al. Azathioprine and hepatic veno-occlusive disease in renal transplant patients. Gastroenterology. 1986; 90:446-454.
- Weitz H, Gokel JM, Loeshke K, et al. Veno-occlusive disease of the liver in patients receiving immunosuppressive therapy. Virchows Arch A Pathol Anat Histol. 1982; 395:245-256.
- Schusziarra V, Ziekursch V, Schlamp R, et al. Pharmacokinetics of azathioprine under haemodialysis. Int J Clin Pharmacol Biopharm. 1976; 14:298-302.
- Recommendations for the safe handling of parenteral antineoplastic drugs. Washington, DC: Division of Safety; Clinical Center Pharmacy Department and Cancer Nursing Services, National Institute of Health; 1992. US Dept of Health and Human Services. Public Health Service Publication NIH 92-2621.
- AMA Council on Scientific Affairs. Guidelines for handling parenteral antineoplastics. JAMA. 1985; 253:1590-1592.
- National Study Commission on Cytotoxic Exposure. Recommendations for handling cytotoxic agents. 1987. Available from Louis P. Jeffrey, Chairman, National Study Commission on Cytotoxic Exposure. Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, MA 02115.
- Clinical Oncological Society of Australia. Guidelines and recommendations for safe handling of antineoplastic agents. Med J Aust. 1983; 1:426-428.
- Jones RB, Frank R, Mass T. Safe handling of chemotherapeutic agents: a report from The Mount Sinai Medical Center. CA Cancer J for Clinicians. 1983; 33:258-263.
- American Society of Hospital Pharmacists. ASHP technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm. 1990; 47:1033-1049.
- Yodaiken RE, Bennett D. OSHA Work-Practice guidelines for personnel dealing with cytotoxic (antineoplastic) drugs. Am J Hosp Pharm, 1996; 43:1193-1204.
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AZATHIOPRINE
azathioprine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-484(NDC:65841-602) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZATHIOPRINE (UNII: MRK240IY2L) (AZATHIOPRINE - UNII:MRK240IY2L) AZATHIOPRINE 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color YELLOW (YELLOW) Score 2 pieces Shape ROUND (ROUND) Size 8mm Flavor Imprint Code ZC;59 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-484-75 75 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077621 07/11/2007 Labeler - Rebel Distributors Corp.. (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp. 118802834 relabel, repack