Label: 200C HOMEOPATHIC REMEDY KIT- 50 remedy kit kit
-
NDC Code(s):
68428-026-01,
68428-028-01,
68428-030-01,
68428-032-01, view more68428-033-01, 68428-034-01, 68428-035-01, 68428-036-01, 68428-040-01, 68428-041-01, 68428-042-01, 68428-045-01, 68428-047-01, 68428-048-01, 68428-049-01, 68428-051-01, 68428-052-01, 68428-053-01, 68428-054-01, 68428-055-01, 68428-056-01, 68428-057-01, 68428-058-01, 68428-059-01, 68428-061-01, 68428-062-01, 68428-064-01, 68428-065-01, 68428-067-01, 68428-068-01, 68428-069-01, 68428-072-01, 68428-073-01, 68428-074-01, 68428-075-01, 71919-759-01, 71919-760-01, 71919-761-01, 71919-762-01, 71919-763-01, 71919-766-01, 71919-767-01, 71919-769-01, 71919-770-01, 71919-771-01, 71919-773-01, 71919-777-01, 71919-812-01, 71919-813-01, 71919-814-01, 71919-822-01, 71919-822-18
- Packager: Washington Homeopathic Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
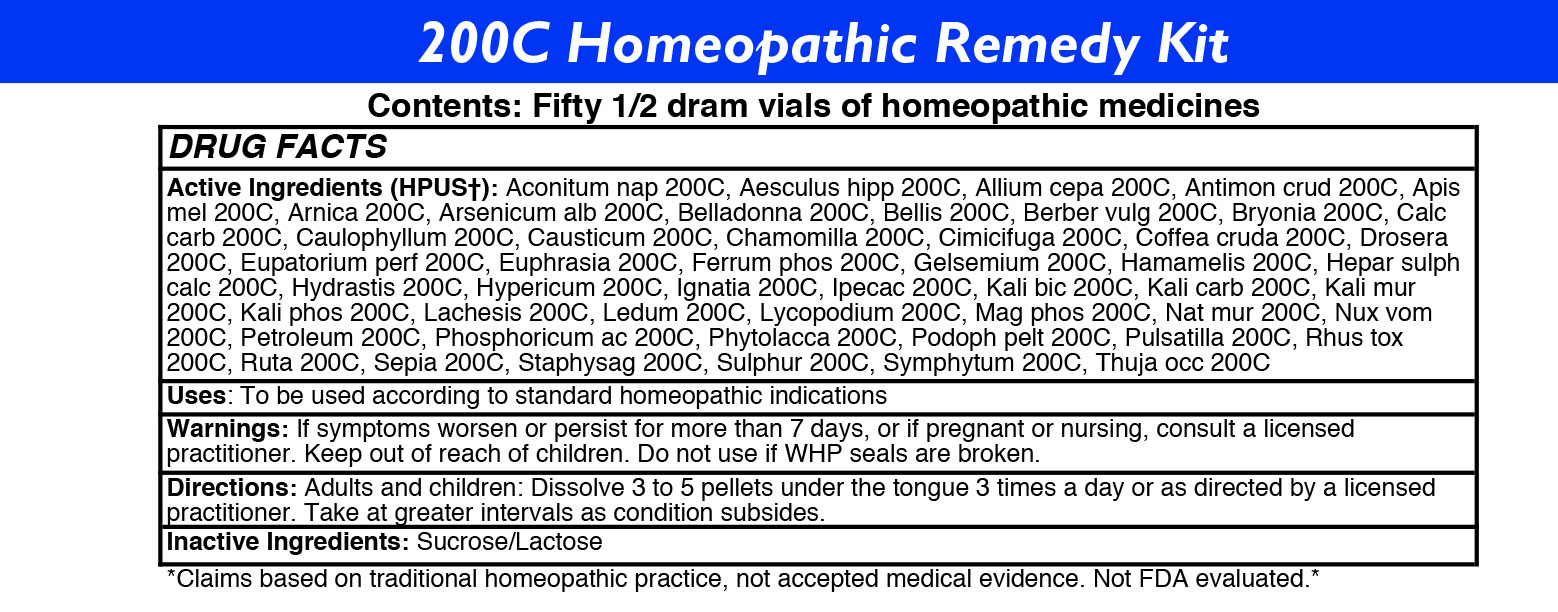
ACTIVE INGREDIENTS
Acon nap
Aesculus hipp
Allium cepa
Antimon crud
Apis mel
Arnica
Arsenicum alb
Belladonna
Bellis
Berber vulg
Bryonia
Calc carb
Caulophyllum
Causticum
Chamomilla
Cimicifuga
Coffea cruda
Drosera
Eupatorium perf
Euphrasia
Ferrum phos
Gelsemium
Hamamelis
Hepar sulph calc
Hydrastis
Hypericum
Ignatia
Ipecac
Kali bic
Kali carb
Kali mur
Kali phos
Lachesis
Ledum
Lycopodium
Mag phos
Nat mur
Nux vom
Petroleum
Phosphoricum ac
Phytolacca
Podoph pelt
Pulsatilla
Rhus tox
Ruta
Sepia
Staphysag
Sulphur
Symphytum
Thuja occ -
USES
To relieve symptoms of fear, hemorrhoids, head cold, warts, stings, bruises, vomiting, fever, bruised soreness, itching, worse motion, oberwork, cramps, hoarseness, irritability, minor back pain, sleeplessness, cough, flu-like symptoms, eye irritation, low fever, lethargy, varicose veins, croupiness, sinuses, shooting pain, sadness, nausea, sinuses, sour belching, congested ears, irritability, sore throat, black eyes, digestion, cramps, sneezing, vomiting, nausea, headache, sore throat, diarrhea, weeping, better motion, bruised feeling, indifference, anger, skin problems, prickling pain, and warts.
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS
Acon nap fear
Aesculus hipp hemorrhoids
Allium cepa head cold
Antimon crud warts
Apis mel stings
Arnica bruises
Arsenicum alb vomiting
Belladonna fever
Bellis bruised soreness
Berber vulg itching
Bryonia worse motion
Calc carb overwork
Caulophyllum cramps
Causticum hoarseness
Chamomilla irritability
Cimicifuga minor back pain
Coffea cruda sleeplessness
Drosera cough
Eupatorium perf flu-like symptoms
Euphrasia eye irritation
Ferrum phos low fever
Gelsemium lethargy
Hamamelis varicose veins
Hepar sulph calc croupiness
Hydrastis sinuses
Hypericum shooting pain
Ignatia sadness
Ipecac nausea
Kali bic sinuses
Kali carb sour belching
Kali mur congested ears
Kali phos irritability
Lachesis sore throat
Ledum black eyes
Lycopodium digestion
Mag phos cramps
Nat mur sneezing
Nux vom vomiting
Petroleum nausea
Phosphoricum ac headache
Phytolacca sore throat
Podoph pelt diarrhea
Pulsatilla weeping
Rhus tox better motion
Ruta bruised feeling
Sepia indifference
Staphysag anger
Sulphur skin problems
Symphytum prickling pain
Thuja occ warts
- STOP USE AND ASK DOCTOR
-
DIRECTIONS
Adults: Dissolve 3 to 5 under the tongue three times a day or as directed by a licensed practitioner. Take at greater intervals as condition subsides.
Children: Dissolve 3 to 5 under the tongue three times a day or as directed by a licensed practitioner. Take at greater intervals as condition subsides.
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
200C HOMEOPATHIC REMEDY KIT
50 remedy kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71919-822 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-822-18 50 in 1 KIT 02/29/2024 1 NDC:71919-822-01 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 750 Part 2 1 VIAL, GLASS 750 Part 3 1 VIAL, GLASS 750 Part 4 1 VIAL, GLASS 350 Part 5 1 VIAL, GLASS 750 Part 6 1 VIAL, GLASS 750 Part 7 1 VIAL, GLASS 350 Part 8 1 VIAL, GLASS 350 Part 9 1 VIAL, GLASS 350 Part 10 1 VIAL, GLASS 750 Part 11 1 VIAL, GLASS 750 Part 12 1 VIAL, GLASS 750 Part 13 1 VIAL, GLASS 750 Part 14 1 VIAL, GLASS 750 Part 15 1 VIAL, GLASS 350 Part 16 1 VIAL, GLASS 750 Part 17 1 VIAL, GLASS 750 Part 18 1 VIAL, GLASS 750 Part 19 1 VIAL, GLASS 750 Part 20 1 VIAL, GLASS 750 Part 21 1 VIAL, GLASS 350 Part 22 1 VIAL, GLASS 750 Part 23 1 VIAL, GLASS 750 Part 24 1 VIAL, GLASS 750 Part 25 1 VIAL, GLASS 750 Part 26 1 VIAL, GLASS 350 Part 27 1 VIAL, GLASS 750 Part 28 1 VIAL, GLASS 350 Part 29 1 VIAL, GLASS 350 Part 30 1 VIAL, GLASS 750 Part 31 1 VIAL, GLASS 750 Part 32 1 VIAL, GLASS 750 Part 33 1 VIAL, GLASS 750 Part 34 1 VIAL, GLASS 750 Part 35 1 VIAL, GLASS 750 Part 36 1 VIAL, GLASS 750 Part 37 1 VIAL, GLASS 750 Part 38 1 VIAL, GLASS 350 Part 39 1 VIAL, GLASS 750 Part 40 1 VIAL, GLASS 750 Part 41 1 VIAL, GLASS 750 Part 42 1 VIAL, GLASS 350 Part 43 1 VIAL, GLASS 750 Part 44 1 VIAL, GLASS 750 Part 45 1 VIAL, GLASS 350 Part 46 1 VIAL, GLASS 750 Part 47 1 VIAL, GLASS 750 Part 48 1 VIAL, GLASS 750 Part 49 1 VIAL, GLASS 750 Part 50 1 VIAL, GLASS 750 Part 1 of 50 HYPERICUM PERF KIT REFILL
hypericum perforatum pelletProduct Information Item Code (Source) NDC:68428-052 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-052-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 2 of 50 NATRUM MURIATICUM KIT REFILL
sodium chloride pelletProduct Information Item Code (Source) NDC:68428-061 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-061-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 3 of 50 MAGNESIA PHOS KIT REFILL
magnesium phosphate, dibasic trihydrate pelletProduct Information Item Code (Source) NDC:68428-059 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-059-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 4 of 50 PETROLEUM KIT REFILL
kerosene pelletProduct Information Item Code (Source) NDC:71919-770 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KEROSENE (UNII: 1C89KKC04E) (KEROSENE - UNII:1C89KKC04E) KEROSENE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-770-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 5 of 50 ARNICA MONTANA KIT REFILL
arnica montana pelletProduct Information Item Code (Source) NDC:68428-032 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-032-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 6 of 50 BELLADONNA KIT REFILL
atropa belladonna pelletProduct Information Item Code (Source) NDC:68428-034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-034-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 7 of 50 KALI PHOSPHORICUM KIT REFILL
potassium phosphate, dibasic pelletProduct Information Item Code (Source) NDC:71919-777 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-777-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/23/2022 Part 8 of 50 PODOPHYLLUM PELT KIT REFILL
podophyllum pelletProduct Information Item Code (Source) NDC:71919-771 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PODOPHYLLUM PELTATUM ROOT (UNII: 2S713A4VP3) (PODOPHYLLUM PELTATUM ROOT - UNII:2S713A4VP3) PODOPHYLLUM PELTATUM ROOT 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-771-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 9 of 50 BERBERIS VULGARIS KIT REFILL
berberis vulgaris root bark pelletProduct Information Item Code (Source) NDC:71919-762 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-762-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/10/2022 Part 10 of 50 CALCAREA CARBONICA KIT REFILL
oyster shell calcium carbonate, crude pelletProduct Information Item Code (Source) NDC:68428-036 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-036-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 11 of 50 EUPHRASIA OFFICINALIS KIT REFILL
euphrasia stricta pelletProduct Information Item Code (Source) NDC:68428-047 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-047-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 12 of 50 COFFEA CRUDA KIT REFILL
arabica coffee bean pelletProduct Information Item Code (Source) NDC:68428-045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-045-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/06/2010 Part 13 of 50 SULPHUR KIT REFILL
sulfur pelletProduct Information Item Code (Source) NDC:68428-073 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-073-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 14 of 50 ALLIUM CEPA KIT REFILL
onion pelletProduct Information Item Code (Source) NDC:68428-028 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-028-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 15 of 50 BELLIS PERENNIS KIT REFILL
bellis perennis pelletProduct Information Item Code (Source) NDC:71919-773 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-773-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/10/2022 Part 16 of 50 FERRUM PHOS KIT REFILL
ferrum phosphoricum pelletProduct Information Item Code (Source) NDC:68428-048 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-048-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 17 of 50 LEDUM PALUSTRE KIT REFILL
ledum palustre twig pelletProduct Information Item Code (Source) NDC:68428-057 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-057-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 18 of 50 RHUS TOX KIT REFILL
toxicodendron radicans leaf pelletProduct Information Item Code (Source) NDC:68428-067 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOXICODENDRON RADICANS LEAF (UNII: CDH3461U7L) (TOXICODENDRON RADICANS LEAF - UNII:CDH3461U7L) TOXICODENDRON RADICANS LEAF 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-067-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 19 of 50 LYCOPODIUM KIT REFILL
lycopodium clavatum spore pelletProduct Information Item Code (Source) NDC:68428-058 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-058-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 20 of 50 PULSATILLA KIT REFILL
pulsatilla vulgaris pelletProduct Information Item Code (Source) NDC:68428-065 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-065-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 21 of 50 EUPATORIUM PERF KIT REFILL
eupatorium perfoliatum flowering top pelletProduct Information Item Code (Source) NDC:71919-766 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-766-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 22 of 50 LACHESIS MUTUS KIT REFILL
lachesis muta venom pelletProduct Information Item Code (Source) NDC:68428-056 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-056-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 23 of 50 GELSEMIUM SEMP KIT REFILL
gelsemium sempervirens root pelletProduct Information Item Code (Source) NDC:68428-049 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-049-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 24 of 50 IGNATIA AMARA KIT REFILL
strychnos ignatii seed pelletProduct Information Item Code (Source) NDC:68428-053 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-053-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 25 of 50 KALI BICHROMICUM KIT REFILL
potassium dichromate pelletProduct Information Item Code (Source) NDC:68428-055 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-055-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 26 of 50 AESCULUS HIPP KIT REFILL
horse chestnut pelletProduct Information Item Code (Source) NDC:71919-759 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-759-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/05/2022 Part 27 of 50 CAUSTICUM KIT REFILL
causticum pelletProduct Information Item Code (Source) NDC:68428-041 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-041-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 28 of 50 DROSERA ROTUNDIFOLIA KIT REFILL
drosera rotundifolia pelletProduct Information Item Code (Source) NDC:71919-761 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-761-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/10/2022 Part 29 of 50 KALI CARBONICUM KIT REFILL
potassium carbonate pelletProduct Information Item Code (Source) NDC:71919-769 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-769-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 30 of 50 KALI MURIATICUM KIT REFILL
potassium chloride pelletProduct Information Item Code (Source) NDC:71919-813 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-813-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/07/2023 Part 31 of 50 ANTIMONIUM CRUDUM KIT REFILL
antimony trisulfide pelletProduct Information Item Code (Source) NDC:71919-812 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-812-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/07/2023 Part 32 of 50 IPECACUANHA KIT REFILL
ipecac pelletProduct Information Item Code (Source) NDC:68428-054 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-054-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 33 of 50 SYMPHYTUM OFF KIT REFILL
comfrey root pelletProduct Information Item Code (Source) NDC:68428-074 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-074-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 34 of 50 THUJA OCCIDENTALIS KIT REFILL
thuja occidentalis leafy twig pelletProduct Information Item Code (Source) NDC:68428-075 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-075-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 35 of 50 ARSENICUM ALBUM KIT REFILL
arsenic trioxide pelletProduct Information Item Code (Source) NDC:68428-033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-033-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 36 of 50 BRYONIA ALBA KIT REFILL
bryonia alba root pelletProduct Information Item Code (Source) NDC:68428-035 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-035-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 37 of 50 CAULOPHYLLUM THAL KIT REFILL
caulophyllum thalictroides root pelletProduct Information Item Code (Source) NDC:68428-040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-040-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 38 of 50 HAMAMELIS VIRGINIANA KIT REFILL
hamamelis virginiana root bark/stem bark pelletProduct Information Item Code (Source) NDC:71919-760 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-760-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/10/2022 Part 39 of 50 NUX VOMICA KIT REFILL
strychnos nux-vomica seed pelletProduct Information Item Code (Source) NDC:68428-062 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-062-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 40 of 50 PHOSPHORICUM ACIDUM KIT REFILL
phosphoric acid pelletProduct Information Item Code (Source) NDC:71919-814 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-814-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/07/2023 Part 41 of 50 PHYTOLACCA KIT REFILL
phytolacca americana root pelletProduct Information Item Code (Source) NDC:68428-064 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-064-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 42 of 50 CIMICIFUGA RACEMOSA KIT REFILL
black cohosh pelletProduct Information Item Code (Source) NDC:71919-767 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-767-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 43 of 50 ACONITUM NAPELLUS KIT REFILL
aconitum napellus pelletProduct Information Item Code (Source) NDC:68428-026 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-026-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 44 of 50 HEPAR SULPH CALC KIT REFILL
calcium sulfide pelletProduct Information Item Code (Source) NDC:68428-051 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-051-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 45 of 50 HYDRASTIS CANADENSIS
goldenseal pelletProduct Information Item Code (Source) NDC:71919-763 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71919-763-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 46 of 50 RUTA GRAVEOLENS KIT REFILL
ruta graveolens flowering top pelletProduct Information Item Code (Source) NDC:68428-068 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-068-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 47 of 50 APIS MELLIFICA KIT REFILL
apis mellifera pelletProduct Information Item Code (Source) NDC:68428-030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-030-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 48 of 50 CHAMOMILLA KIT REFILL
matricaria recutita pelletProduct Information Item Code (Source) NDC:68428-042 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-042-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 49 of 50 SEPIA KIT REFILL
sepia officinalis juice pelletProduct Information Item Code (Source) NDC:68428-069 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-069-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Part 50 of 50 STAPHYSAGRIA KIT REFILL
delphinium staphisagria seed pelletProduct Information Item Code (Source) NDC:68428-072 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 200 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68428-072-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/06/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/29/2024 Labeler - Washington Homeopathic Products (084929389) Establishment Name Address ID/FEI Business Operations Washington Homeopathic Products 084929389 manufacture(71919-822)