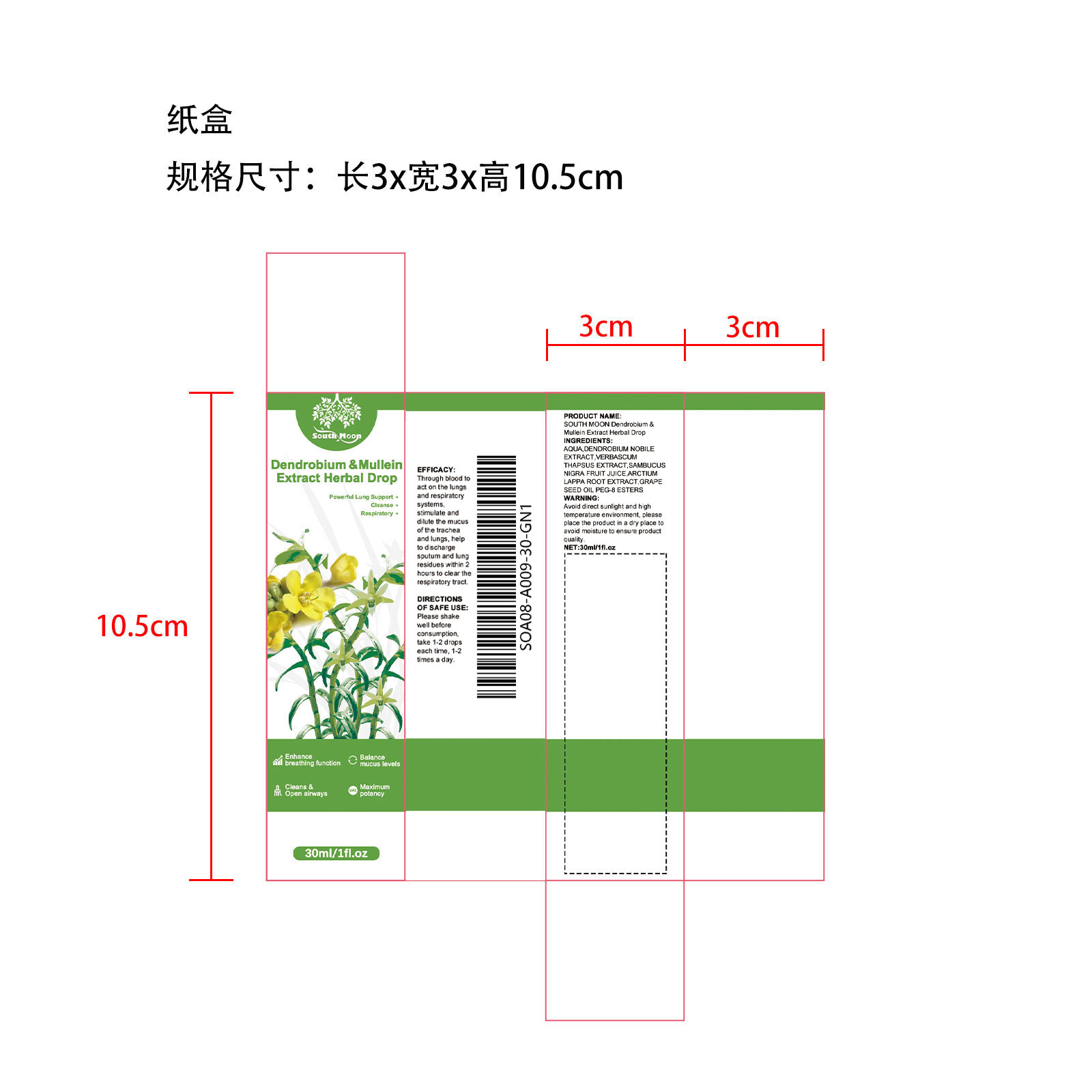
Label: ESSENCE LIQUID liquid
- NDC Code(s): 84067-000-01
- Packager: Shantou Youjia E-Commerce Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ESSENCE LIQUID
essence liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84067-000 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUROPEAN ELDERBERRY JUICE (UNII: Z4IFJ0AK1E) (EUROPEAN ELDERBERRY JUICE - UNII:Z4IFJ0AK1E) EUROPEAN ELDERBERRY JUICE 3 mg in 30 mg VERBASCUM THAPSUS (UNII: C9TD27U172) (VERBASCUM THAPSUS - UNII:C9TD27U172) VERBASCUM THAPSUS 6 mg in 30 mg DENDROBIUM NOBILE STEM (UNII: CGO90PC910) (DENDROBIUM NOBILE STEM - UNII:CGO90PC910) DENDROBIUM NOBILE STEM 6 min in 30 mg GRAPE SEED OIL GLYCERETH-8 ESTERS (UNII: 2NPJ4395VD) (GRAPE SEED OIL GLYCERETH-8 ESTERS - UNII:2NPJ4395VD) GRAPE SEED OIL GLYCERETH-8 ESTERS 3 mg in 30 mg ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) (ARCTIUM LAPPA ROOT - UNII:597E9BI3Z3) ARCTIUM LAPPA ROOT 3 mg in 30 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 9 mg in 30 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84067-000-01 30 mg in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2024 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 02/01/2024 12/31/2024 Labeler - Shantou Youjia E-Commerce Co., Ltd. (711173127) Establishment Name Address ID/FEI Business Operations 711173127 711173127 label(84067-000)