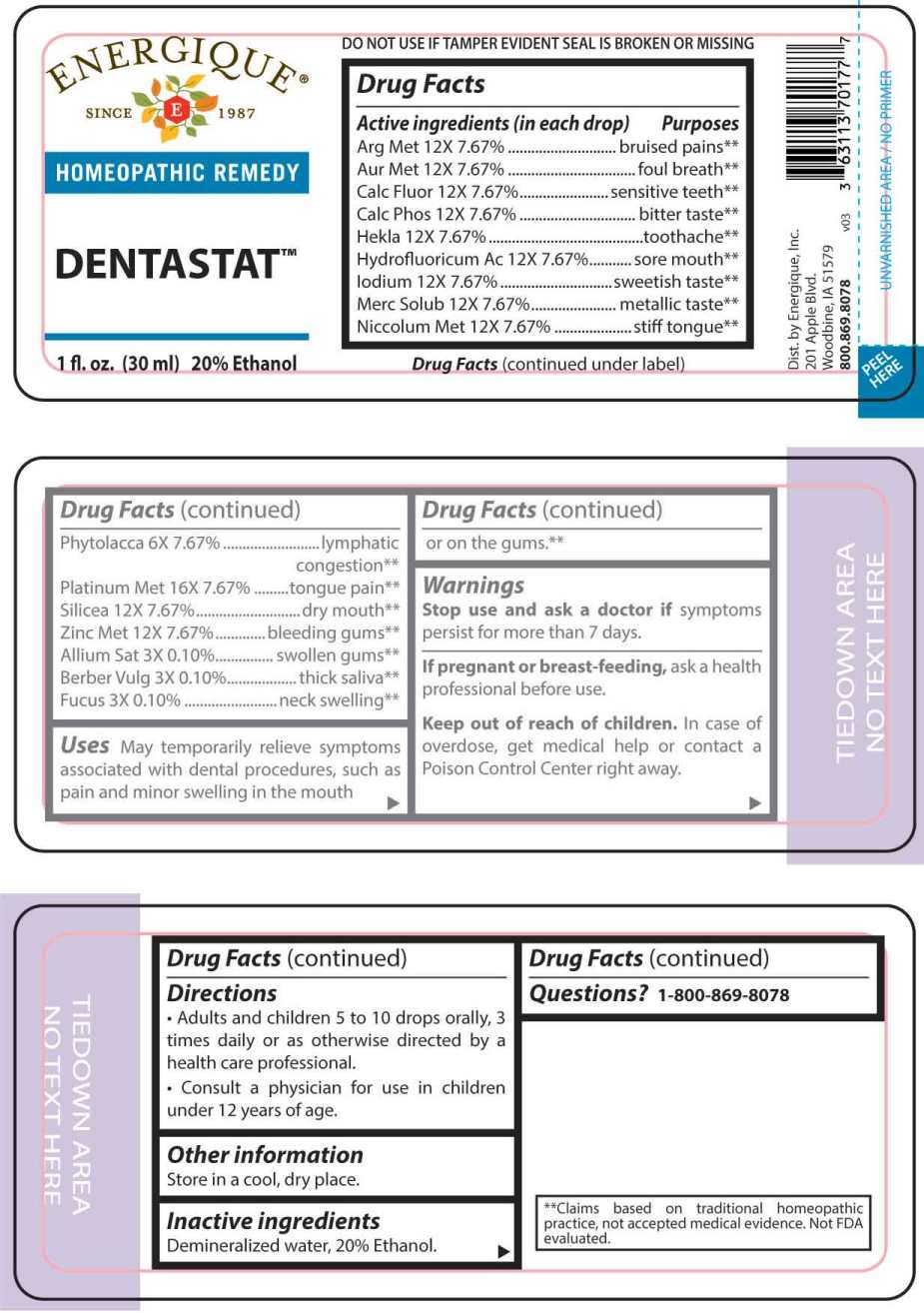
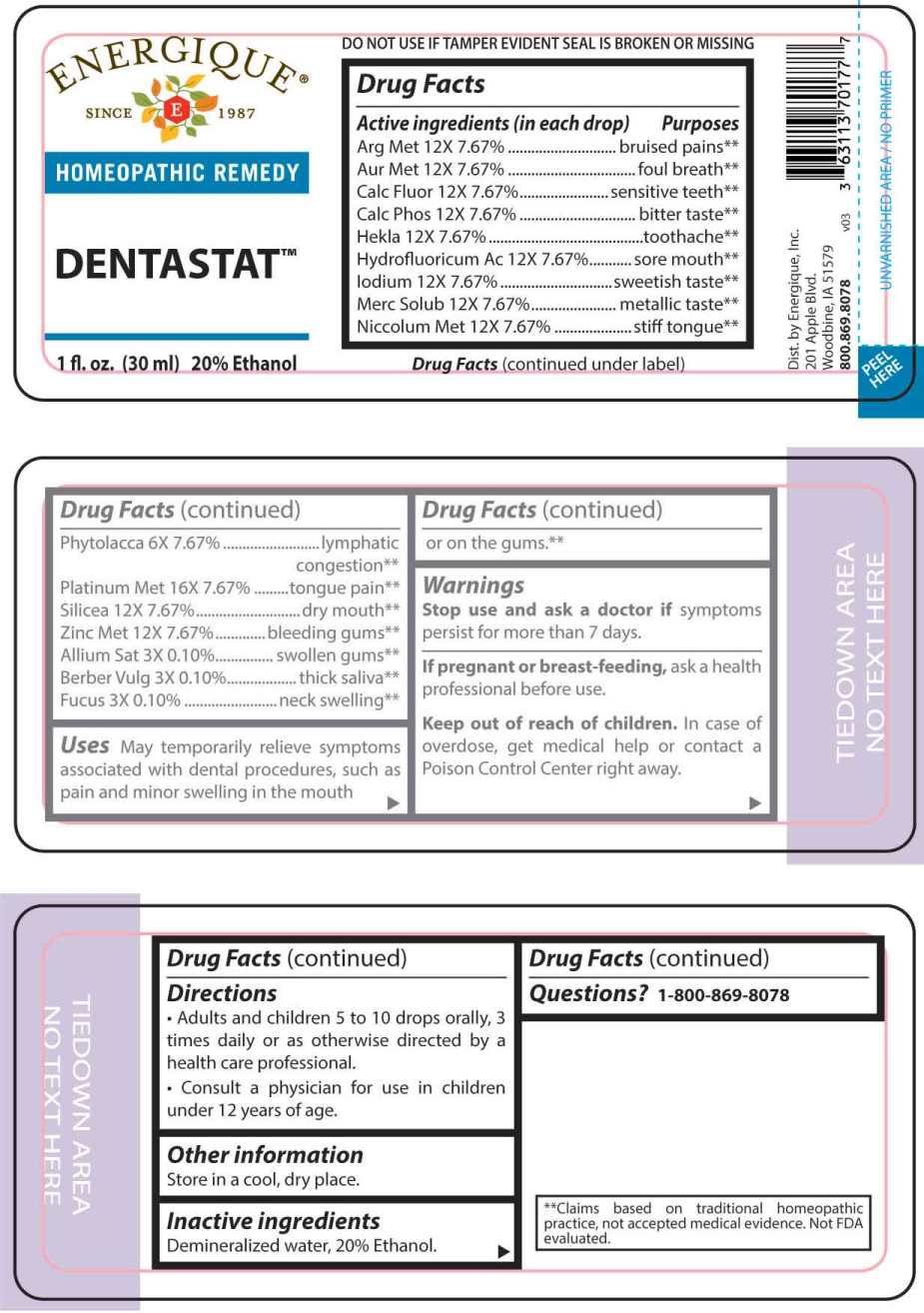
Label: DENTASTAT- allium sativum, berberis vulgaris, fucus vesiculosus, phytolacca decandra, argentum metallicum, aurum metallicum, calcarea fluorica, calcarea phosphorica, hekla lava, hydrofluoricum acidum, iodium, mercurius solubilis, niccolum metallicum, silicea, zincum metallicum, platinum metallicum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 44911-0661-1 - Packager: Energique, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
(in each drop) Argentum Metallicum 12X 7.67%, Aurum Metallicum 12X 7.67%, Calcarea Fluorica 12X 7.67%, Calcarea Phosphorica 12X 7.67%, Hekla Lava 12X 7.67%, Hydrofluoricum Acidum 12X 7.67%, Iodium 12X 7.67%, Mercurius Solubilis 12X 7.67%, Niccolum Metallicum 12X 7.67%, Phytolacca Decandra 6X 7.67%, Platinum Metallicum 16X 7.67%, Silicea 12X 7.67%, Zincum Metallicum 12X 7.67%, Allium Sativum 3X 0.10%, Berberis Vulgaris 3X 0.10%, Fucus Vesiculosus 3X 0.10%.
-
PURPOSES:
Argentum Metallicum – bruised pains**, Aurum Metallicum – foul breath**, Calcarea Fluorica – sensitive teeth**, Calcarea Phosphorica – bitter taste**, Hekla Lava – toothache**, Hydrofluoricum Acidum – sore mouth**, Iodium – sweetish taste**, Mercurius Solubilis – metallic taste**, Niccolum Metallicum – stiff tongue**, Phytolacca Decandra – lymphatic**, Platinum Metallicum – tongue pain**, Silicea - dry mouth**, Zincum Metallicum – bleeding gums**, Allium Sativum – swollen gums**, Berberis Vulgaris – thick saliva**, Fucus Vesiculosus – neck swelling**.
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated. - USES:
-
WARNINGS:
Stop use and ask a doctor if symptoms persist for more than 7 days.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DO NOT USE IF TAMPER EVIDENT SEAL IS BROKEN OR MISSING. Store in a cool, dry place.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
DENTASTAT
allium sativum, berberis vulgaris, fucus vesiculosus, phytolacca decandra, argentum metallicum, aurum metallicum, calcarea fluorica, calcarea phosphorica, hekla lava, hydrofluoricum acidum, iodium, mercurius solubilis, niccolum metallicum, silicea, zincum metallicum, platinum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44911-0661 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 3 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 3 [hp_X] in 1 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 3 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 6 [hp_X] in 1 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 1 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 1 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] in 1 mL HEKLA LAVA (UNII: C21158IIRK) (HEKLA LAVA - UNII:C21158IIRK) HEKLA LAVA 12 [hp_X] in 1 mL HYDROFLUORIC ACID (UNII: RGL5YE86CZ) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 12 [hp_X] in 1 mL IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 12 [hp_X] in 1 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 12 [hp_X] in 1 mL NICKEL (UNII: 7OV03QG267) (NICKEL - UNII:7OV03QG267) NICKEL 12 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 12 [hp_X] in 1 mL PLATINUM (UNII: 49DFR088MY) (PLATINUM - UNII:49DFR088MY) PLATINUM 16 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44911-0661-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/06/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/06/2022 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0661) , api manufacture(44911-0661) , label(44911-0661) , pack(44911-0661)