Label: VETRIMEC PLUS- ivermectin and clorsulon injection
- NDC Code(s): 13985-550-10, 13985-550-50
- Packager: VetOne
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 16, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Approved by FDA under ANADA #200-450
Restricted Drug (California)-Use Only as Directed
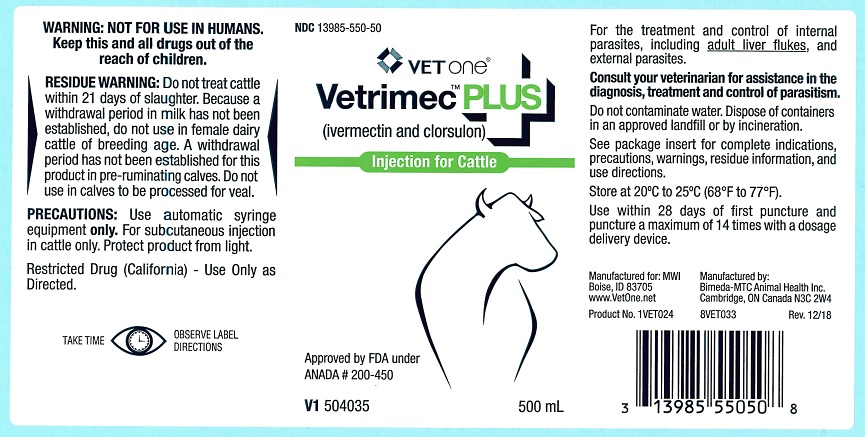
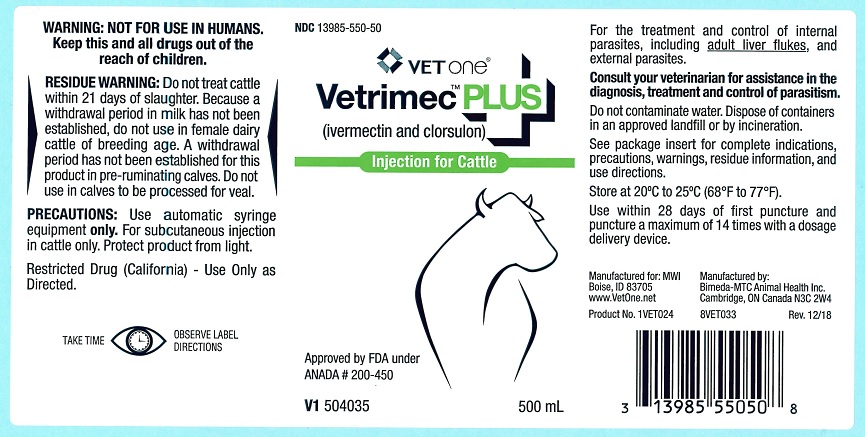
Vetrimec™ Plus
(ivermectin and clorsulon)
Injection for Cattle
For the effective treatment and control of internal parasites, including adult liver flukes, and external parasites.
Consult your veterinarian for assistance in the diagnosis, treatment and control of parasitism.
INTRODUCTION: The ability of ivermectin to deliver internal and external parasite control has been proven in cattle markets around the world. Now, Vetrimec™ Plus combines ivermectin, the active ingredient of Vetrimec™ 1%, with clorsulon, an effective adult flukicide. A single injection of Vetrimec™ Plus (ivermectin and clorsulon) offers all the benefts of Vetrimec™ 1% plus control of adult Fasciola hepatica.
The dosage level of clorsulon supplied by Vetrimec™ Plus is effective only against adult liver flukes (Fasciola hepatica).
PRODUCT DESCRIPTION: Vetrimec™ Plus is a ready-to-use sterile solution containing 1% w/v ivermectin, 10% w/v clorsulon, 40% glycerol formal, and propylene glycol, q.s. ad 100%. It is formulated to deliver the recommended dose level of 200 mcg ivermectin/kg and 2 mg clorsulon/kg given subcutaneously behind the shoulder at the rate of 1 mL per 110 lb (50 kg) body weight.
-
MECHANISM OF ACTION
MODE OF ACTION: Ivermectin is a member of the macrocyclic lactone class of endectocides which have a unique mode of action. Compounds of the class bind selectively and with high affnity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or
muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated chloride channels, the macrocyclic lactones have a low affnity for other mammalian ligand-gated chloride channels and they do not readily cross the blood-brain barrier. Clorsulon is rapidly absorbed into the circulating blood. Erythrocytes with bound drug as well as plasma are ingested by Fasciola hepatica. Adult Fasciola hepatica are killed by clorsulon because of inhibition of enzymes in the glycolytic pathway, which is their primary source of energy. -
INDICATIONS & USAGE
INDICATIONS: Vetrimec™ Plus is indicated for the effective treatment and control of the following parasites in cattle:
Gastrointestinal Roundworms (adults and fourth-stage larvae):
Ostertagia ostertagi (including inhibited O. ostertagi)O. lyrata
Haemonchus placei
Trichostrongylus axei
T. colubriformis
Cooperia oncophora
C. punctata
C. pectinata
Bunostomum phlebotomum
Nematodirus helvetianus (adults only)
N. spathiger (adults only)
Oesophagostomum radiatum
Lungworms (adults and fourth-stage larvae):
Dictyocaulus viviparusLiver Flukes:
Fasciola hepatica (adults only)Cattle Grubs (parasitic stages):
Hypoderma bovis
H. lineatum
Sucking Lice:
Linognathus vituli
Haematopinus eurysternus
Solenopotes capillatus
Mange Mites (Cattle Scab*):
Psoroptes ovis (syn. P. communis var. bovis)
Sarcoptes scabiei var. bovis
Persistent Activity: Ivermectin and clorsulon injection has been proved to effectively control infections and to protect cattle from reinfection with Dictyocaulus viviparus and Oesophagostomum radiatum for 28 days after treatment; Ostertagia ostertagi, Trichostrongylus axei and Cooperia punctata for 21 days after treatment; Haemonchus placei and Cooperia oncophora for 14 days after treatment.
*Ivermectin has been approved as a scabicide by USDA/APHIS. Federal regulations require that cattle infested with or exposed to scabies (i.e., infestations with Psoroptes ovis) be treated. Ivermectin when used according to label instructions meets this requirement. Treated cattle may be shipped interstate, but they must not be mixed with other cattle for 14 days following treatment. The federal regulations make no restriction on the movement of cattle not affected with or exposed to scabies. However, individual states have additional regulations to govern the interstate shipment of cattle and the regulatory veterinarian in the state of destination should be consulted for
applicable regulations on the use of ivermectin in the control of scabies. -
DOSAGE & ADMINISTRATION
DOSAGE:Vetrimec™ Plus should be given only by subcutaneous injection at a dose volume of 1 mL per 110 lb. (50 kg) body weight. This volume will deliver 10 mg ivermectin and 100 mg clorsulon. For example:
Body Weight (lb) Dose Volume (mL) 220 2 330 3 440 4 550 5 660 6 770 7 880 8 990 9 1100 10 ADMINISTRATION: Vetrimec™ Plus (ivermectin and clorsulon) is to be given subcutaneously only. Animals should be appropriately restrained to achieve the proper route of administration. Use of a 16-gauge, ½” to ¾” sterile needle is recommended. Inject the solution subcutaneously (under the skin) behind the shoulder (see illustration).
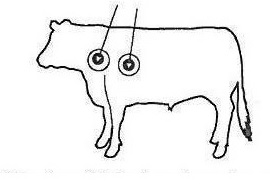
When using the 500 mL or 1000 mL package size, use only automatic syringe equipment.
Use sterile equipment and sanitize the injection site by applying a suitable disinfectant. Clean, properly disinfected needles should be used to reduce the potential for injection site infections.
No special handling or protective clothing is necessary.
The viscosity of the product increases in cool temperatures. Andministering Vetrimec™ Plus at temperatures of 5°C (41°F) or below may be difficult. Users can make dosing easier by warming both the product and the injection equipment to about 15°C (59°F).
Use within 28 days of first puncture and puncture a maximum of 14 times with a dosage delivery device.
ANIMAL SAFETY: In breeding animals (bulls and cows), ivermectin and clorsulon used at the recommended level had no effect on breeding performance.
-
RESIDUE WARNING
WARNING: NOT FOR USE IN HUMANS.
Keep this and all drugs out of reach of children.
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report adverse effects, obtain an SDS or for assistance, contact Bimeda, Inc. at 1-888-524-6332.RESIDUE WARNING: Do not treat cattle within 21 days of slaughter. Because a withdrawal period in milk has not been established, do not use in female dairy cattle of breeding age. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
-
PRECAUTIONS
PRECAUTIONS: Transitory discomfort has been observed in some cattle following subcutaneous administration. Soft tissue swelling at the injection site has also been observed. These reactions have disappeared without treatment. Divide doses greater than 10 mL between two injection sites to reduce occasional discomfort or site reaction. Different injection sites should be used for other parenteral products.
Vetrimec™ Plus has been developed specifcally for use in cattle only. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
For subcutaneous injection in cattle only.
This product is not for intravenous or intramuscular use.
Restricted Drug (California) - Use Only as Directed.
When to Treat Cattle with Grubs: Vetrimec™ Plus effectively controls all stages of cattle grubs. However, proper timing of treatment is important. For most effective results, cattle should be treated as soon as possible after the end of the heel fy (warble fy) season.
Destruction of Hypoderma larvae (cattle grubs) at the period when these grubs are in vital areas may cause undesirable host-parasite reactions including the possibility of fatalities. Killing Hypoderma lineatum when it is in the tissue surrounding the esophagus (gullet) may cause bloat; killing H. bovis when it is in the vertebral canal may cause staggering or paralysis. These reactions are not specifc to treatment with Vetrimec™ Plus, but can occur with any successful treatment of grubs. Cattle should be treated either before or after stages of grub development. Consult your veterinarian concerning the proper time for treatment.
Cattle treated with Vetrimec™ Plus after the end of the heel fly season may be retreated with ivermectin during the winter for internal parasites, mange mites, or sucking lice, without danger of grub-related reactions. A planned parasite control program is recommended.
Protect product from light.
Environmental Safety: Studies indicate that when ivermectin comes in contact with soil, it readily and tightly binds to the soil and becomes inactive over time. Free ivermectin may adversely affect fish and certain aquatic organisms. Do not permit water runoff from feedlots to enter lakes,streams or ponds. Do not contaminate water by direct application or by improper disposal of drug containers. Dispose of containers in an approved landfill or by incineration.
As with other avermectins, ivermectin is excreted in the dung of treated animals and can inhibit the reproduction and growth of pest and beneficial insects that use dung as a source of food and for reproduction. The magnitude and duration of such effects are species and life-cycle specific. When used according to label directions, the product is not expected to have an adverse impact on populations of dung-dependent insects.
-
HOW SUPPLIED
HOW SUPPLIED: Vetrimec™ Plus is available in two ready-to-use sizes:
The 500 mL plastic bottle is suitable for use with automatic syringe equipment. Each bottle contains suffcient solution to treat 100 head of 550 lb (250 kg) cattle.
The 1000 mL plastic bottle is suitable for use with automatic syringe equipment. Each bottle contains suffcient solution to treat 200 head of 550 lb (250 kg) cattle.
- STORAGE AND HANDLING
- 500mL
-
INGREDIENTS AND APPEARANCE
VETRIMEC PLUS
ivermectin and clorsulon injectionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:13985-550 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 10 mg in 1 mL CLORSULON (UNII: EG1ZDO6LRD) (CLORSULON - UNII:EG1ZDO6LRD) CLORSULON 100 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-550-50 500 mL in 1 VIAL, MULTI-DOSE 2 NDC:13985-550-10 1000 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200450 03/28/2019 Labeler - VetOne (019926120) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda- MTC 256232216 manufacture Establishment Name Address ID/FEI Business Operations Aino Co., Ltd. of North China Pharmaceutical Group 529804676 api manufacture Establishment Name Address ID/FEI Business Operations Alivira Animal Health Limited 650916617 api manufacture Establishment Name Address ID/FEI Business Operations Shandong Qilu King-phar Pharmaceutical Co., Ltd. 421524323 api manufacture