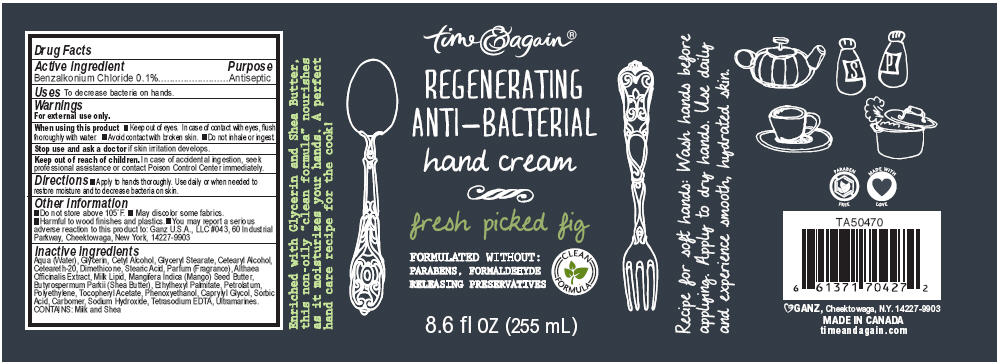
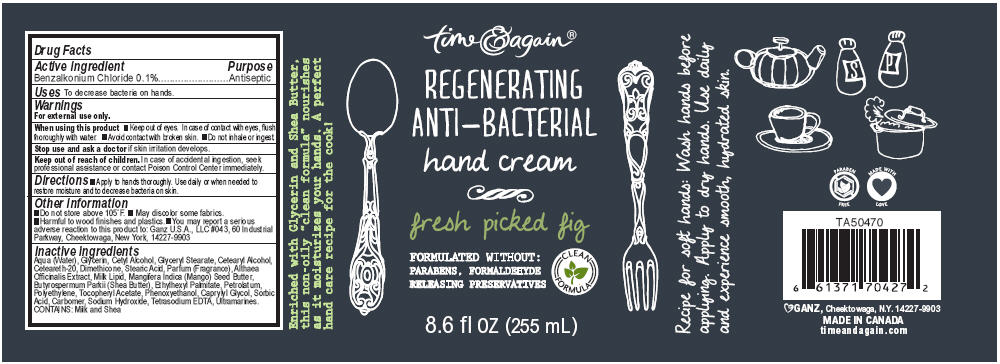
Label: GOURMET KITCHEN REGENERATING ANTI-BACTERIAL FRESH PICKED FIG- benzalkonium chloride lotion
GOURMET KITCHEN REGENERATING ANTI-BACTERIAL SWEET PLUM- benzalkonium chloride lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 75862-019-01, 75862-023-01 - Packager: GANZ U.S.A., LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (Water), Glycerin, Cetyl Alcohol, Glyceryl Stearate, Cetearyl Alcohol, Ceteareth-20, Dimethicone, Stearic Acid, Parfum (Fragrance), Althaea Officinalis Extract, Milk Lipid, Mangifera Indica (Mango) Seed Butter, Butyrospermum Parkii (Shea Butter), Ethylhexyl Palmitate, Petrolatum, Polyethylene, Tocopheryl Acetate, Phenoxyethanol, Caprylyl Glycol, Sorbic Acid, Carbomer, Sodium Hydroxide, Tetrasodium EDTA, Ultramarines. CONTAINS: Milk and Shea
- PRINCIPAL DISPLAY PANEL - 255 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 255 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
GOURMET KITCHEN REGENERATING ANTI-BACTERIAL FRESH PICKED FIG
benzalkonium chloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75862-019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) (Benzalkonium - UNII:7N6JUD5X6Y) Benzalkonium Chloride 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Cetyl Alcohol (UNII: 936JST6JCN) Glyceryl Monostearate (UNII: 230OU9XXE4) Cetostearyl Alcohol (UNII: 2DMT128M1S) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Dimethicone (UNII: 92RU3N3Y1O) Stearic Acid (UNII: 4ELV7Z65AP) Althaea Officinalis Root (UNII: TRW2FUF47H) Cow Milk Fat (UNII: 463JZS0XJ3) Mangifera Indica Seed Butter (UNII: 4OXD9M35X2) Shea Butter (UNII: K49155WL9Y) Ethylhexyl Palmitate (UNII: 2865993309) Petrolatum (UNII: 4T6H12BN9U) High Density Polyethylene (UNII: UG00KM4WR7) Alpha-Tocopherol Acetate (UNII: 9E8X80D2L0) Phenoxyethanol (UNII: HIE492ZZ3T) Caprylyl Glycol (UNII: 00YIU5438U) Sorbic Acid (UNII: X045WJ989B) Carbomer Homopolymer Type C (UNII: 4Q93RCW27E) Sodium Hydroxide (UNII: 55X04QC32I) Edetate Sodium (UNII: MP1J8420LU) Ultramarine Blue (UNII: I39WR998BI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75862-019-01 255 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333E 11/30/2011 GOURMET KITCHEN REGENERATING ANTI-BACTERIAL SWEET PLUM
benzalkonium chloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75862-023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) (Benzalkonium - UNII:7N6JUD5X6Y) Benzalkonium Chloride 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Cetyl Alcohol (UNII: 936JST6JCN) Glyceryl Monostearate (UNII: 230OU9XXE4) Cetostearyl Alcohol (UNII: 2DMT128M1S) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Dimethicone (UNII: 92RU3N3Y1O) Stearic Acid (UNII: 4ELV7Z65AP) Althaea Officinalis Root (UNII: TRW2FUF47H) Cow Milk Fat (UNII: 463JZS0XJ3) Mangifera Indica Seed Butter (UNII: 4OXD9M35X2) Shea Butter (UNII: K49155WL9Y) Ethylhexyl Palmitate (UNII: 2865993309) Petrolatum (UNII: 4T6H12BN9U) High Density Polyethylene (UNII: UG00KM4WR7) Alpha-Tocopherol Acetate (UNII: 9E8X80D2L0) Phenoxyethanol (UNII: HIE492ZZ3T) Caprylyl Glycol (UNII: 00YIU5438U) Sorbic Acid (UNII: X045WJ989B) Carbomer Homopolymer Type C (UNII: 4Q93RCW27E) Sodium Hydroxide (UNII: 55X04QC32I) Edetate Sodium (UNII: MP1J8420LU) Ultramarine Blue (UNII: I39WR998BI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75862-023-01 255 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333E 11/30/2011 Labeler - GANZ U.S.A., LLC (798785242)