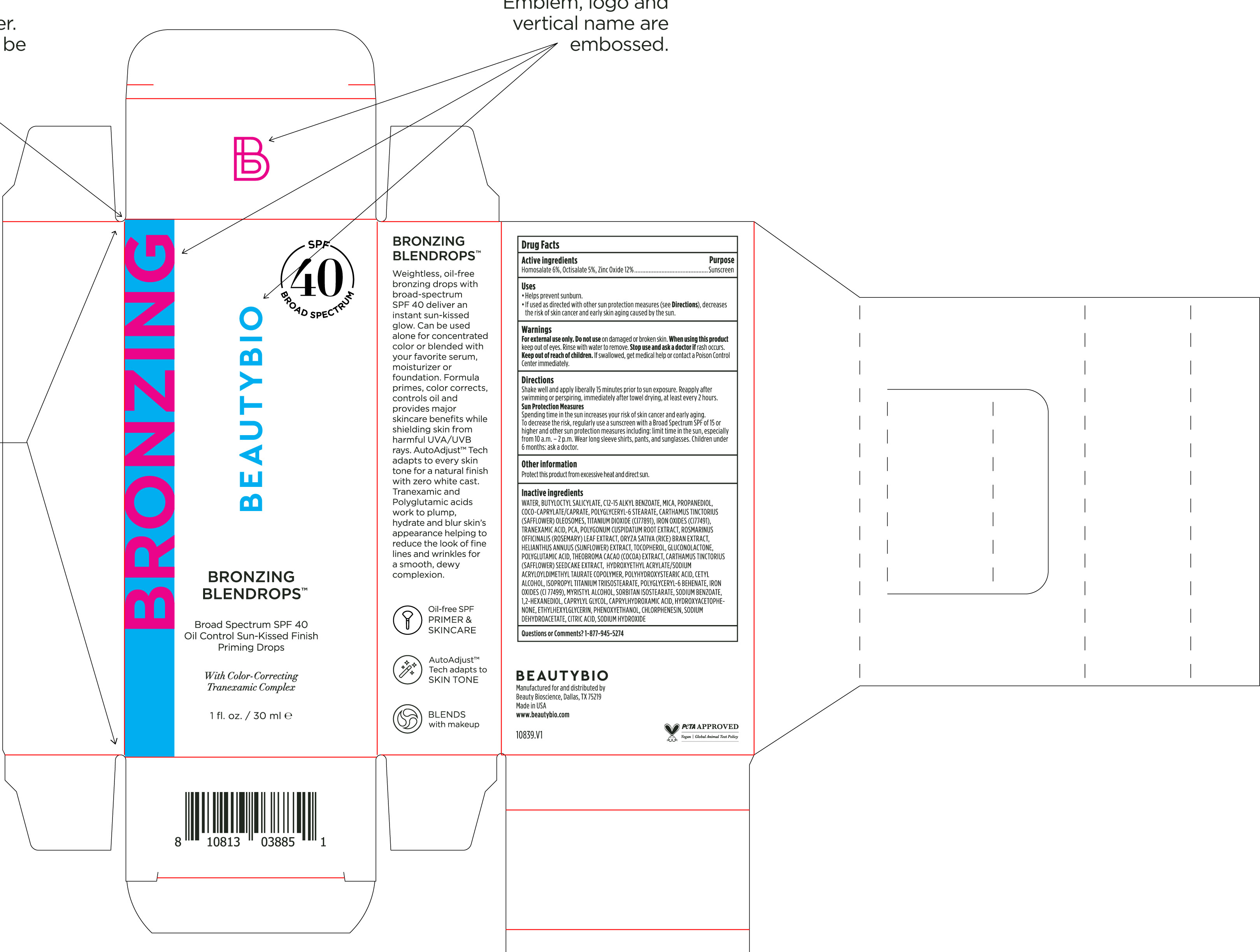
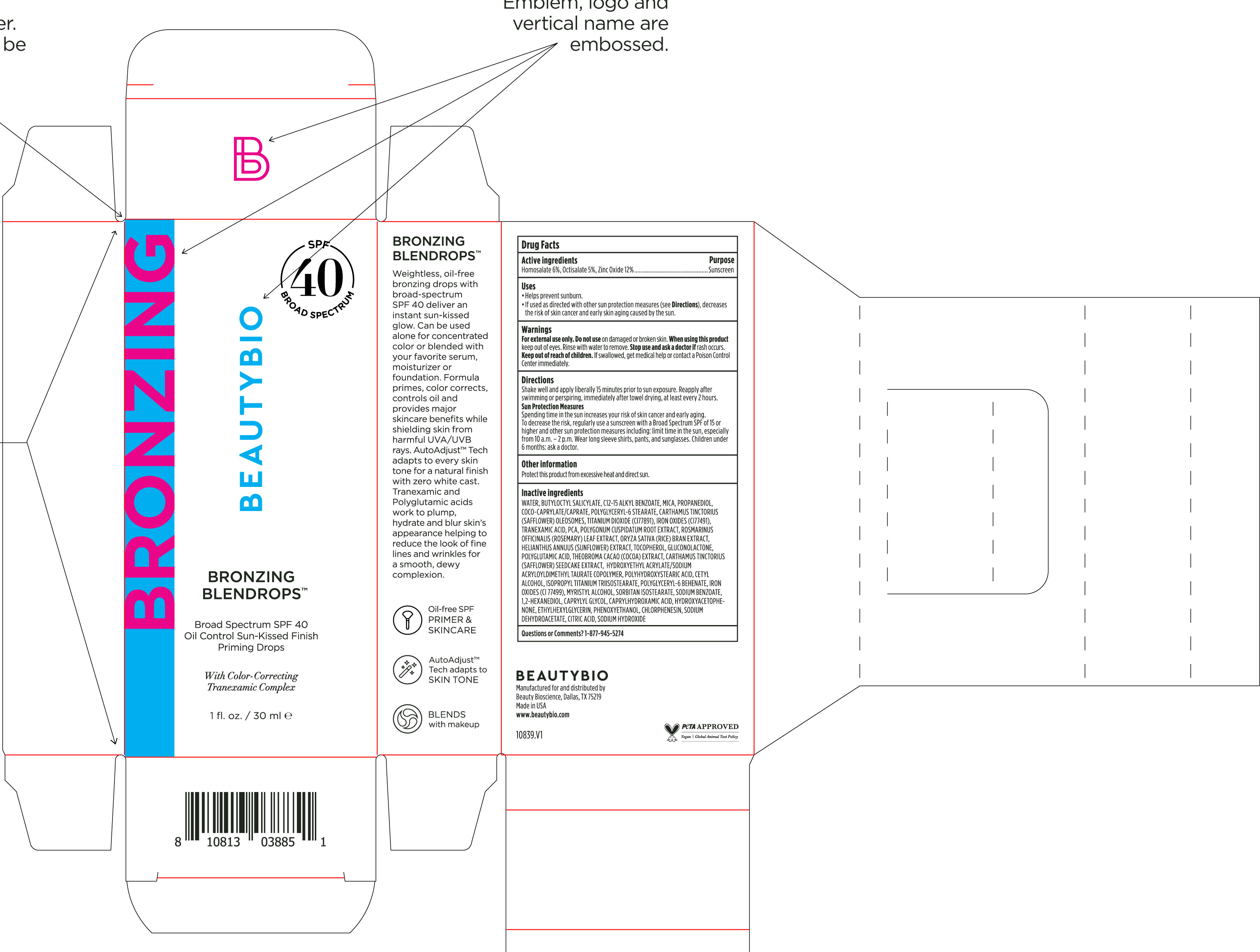
Label: BRONZING BLENDROPS BROAD SPECTRUM SPF 40- homosalate, octisalate, zinc oxide solution/ drops
- NDC Code(s): 44717-082-01
- Packager: Wasatch Product Development, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
Shake well and apply liberally 15 minutes before sun exposure. Reapply after swimming or perspiring, immediately after towel drying, at least every 2 hours.
Sun Protection MeasuresSpending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m. Wear long-sleeve shirts, pants, hats, and sunglasse. Children under 6 months: Ask a doctor - OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Water, Butyloctyl Salicylate, C12-15 Alkyl Benozate, Mica, Propanediol, Coco-Caprylate/Caprate, Polyglyceryl-6 Stearate, Carthamus Tinctorius (Safflower) Oleosomes, Titanium Dioxide (CI77891), Iron Oxides (CI 77491), Tranexamic Acid, PCA, Polygonum Cuspidatum Root Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Oryza Sativa (Rice) Bran Extract, Helianthus Annuus (Sunflower) Extract, Tocopherol, Gluconolactone, Polyglutamic Acid, Theobroma Cacao (Cocoa) Extract, Carthamus Tinctorius (Safflower) Seedcake Extract, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polyhydroxystearic Acid, Cetyl Alcohol, Isopropyl Titanium Triisostearate, Polyglyceryl-6 Behenate, Iron Oxides (CI 77499), Myristyl Alcohol, Sorbitan Isostearate, Sodium Benzoate, 1,2-Hexanediol, Caprylyl Glycol, Caprylhydroxamic Acid, Hydroxyacetophenone, Ethylhexylglycerin, Phenoxyethanol, Chlorphenesin, Sodium Dehydroacetate, Citric Acid, Sodium Hydroxide
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRONZING BLENDROPS BROAD SPECTRUM SPF 40
homosalate, octisalate, zinc oxide solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44717-082 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 6 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 12 g in 100 mL Inactive Ingredients Ingredient Name Strength HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) POLYGLYCERYL-6 BEHENATE (UNII: 4T2L7QI313) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) ROSEMARY (UNII: IJ67X351P9) RICE BRAN (UNII: R60QEP13IC) GLUCONOLACTONE (UNII: WQ29KQ9POT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) POLYGLYCERYL-6 STEARATE (UNII: ETY9Q81E2T) TRANEXAMIC ACID (UNII: 6T84R30KC1) CETYL ALCOHOL (UNII: 936JST6JCN) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) COCOA (UNII: D9108TZ9KG) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) REYNOUTRIA JAPONICA ROOT (UNII: 7TRV45YZF7) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CARTHAMUS TINCTORIUS SEEDCAKE (UNII: DHQ13F4N2W) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) PROPANEDIOL (UNII: 5965N8W85T) PIDOLIC ACID (UNII: SZB83O1W42) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) MYRISTYL ALCOHOL (UNII: V42034O9PU) SODIUM BENZOATE (UNII: OJ245FE5EU) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44717-082-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/02/2024 Labeler - Wasatch Product Development, LLC (962452533)