Label: NARCAN NALOXONE HCL spray
- NDC Code(s): 51662-1642-1, 51662-1642-2
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 69547-627
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (IN EACH SPRAY)
- PURPOSE
- USES
- WARNINGS
-
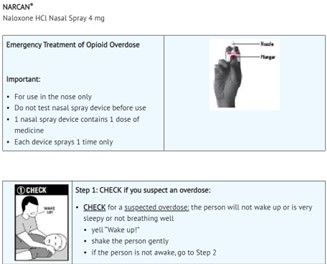
DIRECTIONS

For opioid emergencies, call 911. For questions on NARCAN, call 1-844-4NARCAN (1-844-462-7226) or go to WWW.NARCAN.COM.
©2023 Emergent Devices Inc. EMERGENT® and NARCAN® are registered trademarks of Emergent BioSolutions Inc, or its subsidiaries.
Other Information
•
store at room temperature or refrigerated, between 2°C to 25°C (36°F to 77°F)
•
do not freeze
•
avoid excessive heat above 40°C (104°F)
•
protect from light
•
the product is packaged in individually-sealed blisters.Do not use if the blister is open or torn, or if the device appears damaged.
- INACTIVE INGREDIENT
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
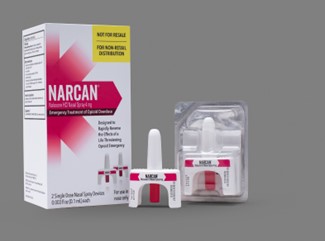
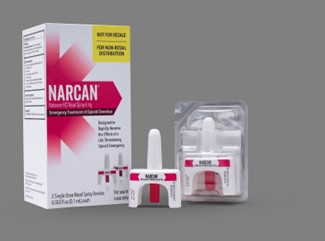
- PRINCIPAL DISPLAY PANEL- CARTON LABEL
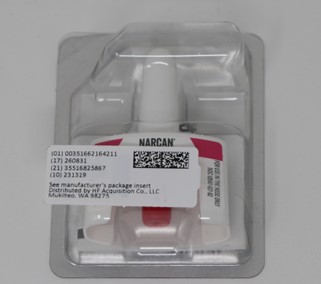
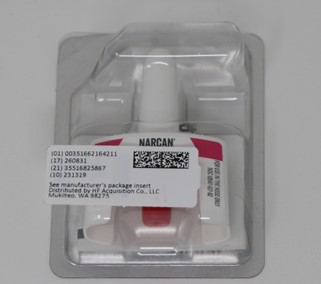
- PRINCIPAL DISPLAY PANEL_ NDC 51662-1642-1
- PRINCIPAL DISPLAY PANEL- NDC 51662-1642-2
-
INGREDIENTS AND APPEARANCE
NARCAN NALOXONE HCL
narcan naloxone hcl sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51662-1642(NDC:69547-627) Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1642-2 2 in 1 CARTON 08/28/2023 1 NDC:51662-1642-1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208411 08/28/2023 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1642)