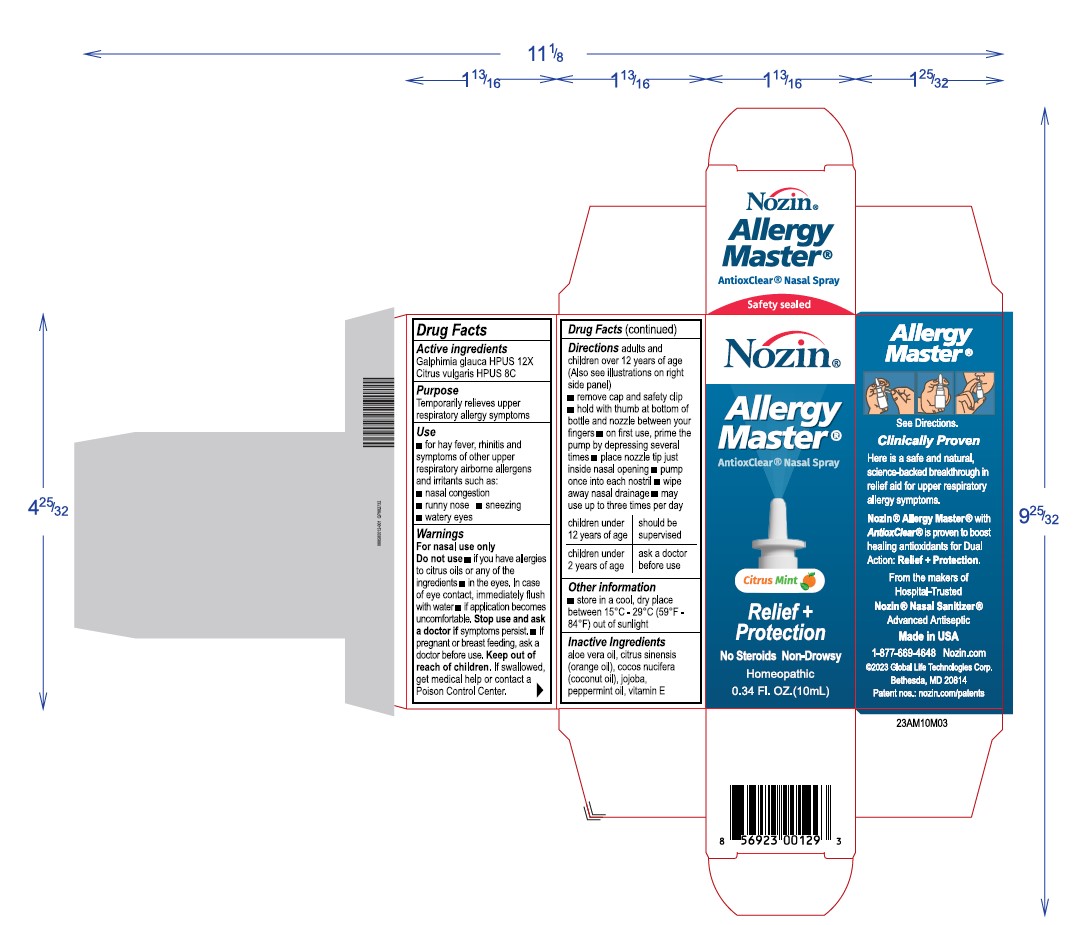
Label: NOZIN ALLERGY MASTER- galphimia glauca, citrus vulgaris spray
- NDC Code(s): 72363-028-03
- Packager: AG Essence
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Directions
Adults and childrenover 12 years of age (Also see illustrations on right side)
- remove cap and safety clip
- hold with thumb at bottomof bottles and nozzle between your fingers
- on first use, prime the pump by depressing several times
- place nozzle tip to just inside nasal opening
- pimp once into each nostril
- wipe away nasal drainage
- may use up to 3 times per day
children under 12 years of age shgould be supervised
children under 2 years of age ask a doctor before use
- Other Information
- Active Ingredients
- Inactive Ingredients
- Purpose
-
Warnings
For nasal use only
Do not use
if you have allergies to citrus oild or any of the other ingredients
in the eyes, in case of eye contact, immediately flus with water
if application becomes uncomfortable Stop use and ask a doctor if symptoms persist
if pregnant or breast feeding, ask a doctor before use. - Keep out of reach of children
- Use
- Nozin Allergy Master box
-
INGREDIENTS AND APPEARANCE
NOZIN ALLERGY MASTER
galphimia glauca, citrus vulgaris sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72363-028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GALPHIMIA GLAUCA WHOLE (UNII: RW4TS05W0D) (GALPHIMIA GLAUCA WHOLE - UNII:RW4TS05W0D) GALPHIMIA GLAUCA WHOLE 0.001 g in 10 g CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) (CITRUS AURANTIUM FRUIT OIL - UNII:59JDQ5VT0T) CITRUS AURANTIUM FRUIT OIL 0.001 g in 10 g Inactive Ingredients Ingredient Name Strength ALOE VERA WHOLE (UNII: KIZ4X2EHYX) 0.49 g in 10 g PEPPERMINT OIL (UNII: AV092KU4JH) 0.075 g in 10 g VITAMIN E POLYETHYLENE GLYCOL SUCCINATE (UNII: O03S90U1F2) 0.025 g in 10 g ORANGE OIL (UNII: AKN3KSD11B) 0.49 g in 10 g JOJOBA OIL (UNII: 724GKU717M) 6.92 g in 10 g COCONUT OIL (UNII: Q9L0O73W7L) 2 g in 10 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72363-028-03 1 in 1 BOX 03/04/2024 1 10 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/04/2024 Labeler - AG Essence (068562165) Registrant - AG Essence (068562165) Establishment Name Address ID/FEI Business Operations AG Essence 068562165 manufacture(72363-028)