Label: ROSEMARY OIL liquid
- NDC Code(s): 83872-021-01
- Packager: Shenzhen XiaoMai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
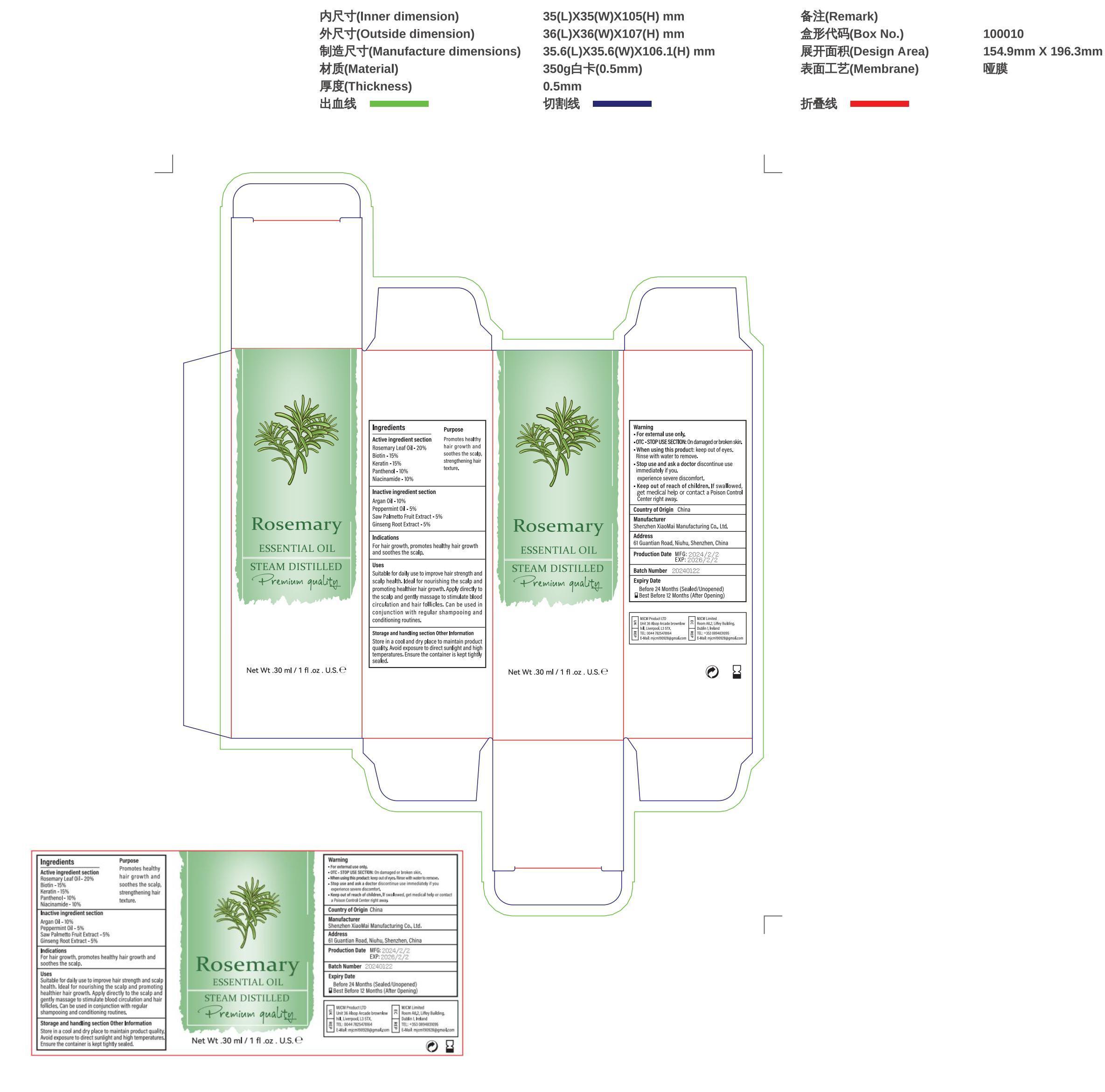
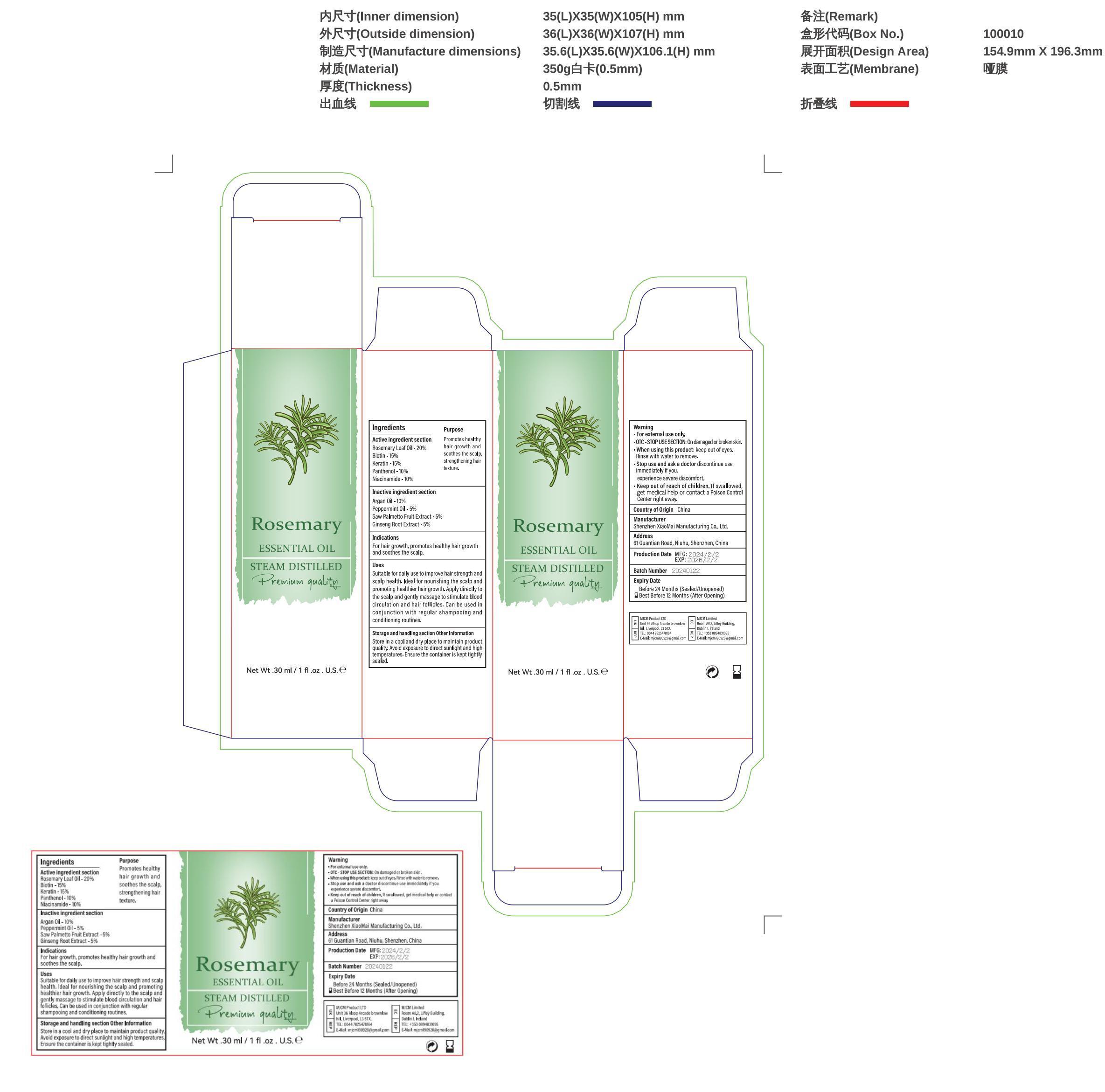
- Active ingredients
- Purpose
-
Uses
Suitable for daily use to improve hair strength and scalp health.
Ideal for nourishing the scalp and promoting healthier hair growth.
Apply directly to the scalp and gently massage to stimulate blood circulation and hair follicles.
Can be used in conjunction with regular shampooing and conditioning routines.
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
Suitable for daily use to improve hair strength and scalp health.
Ideal for nourishing the scalp and promoting healthier hair growth.
Apply directly to the scalp and gently massage to stimulate blood circulation and hair follicles.
Can be used in conjunction with regular shampooing and conditioning routines. - Inactive ingredients
- Other information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROSEMARY OIL
rosemary oil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 10 mg in 100 g HAIR KERATIN AMINO ACIDS (UNII: G46579QK1M) (HAIR KERATIN AMINO ACIDS - UNII:G46579QK1M) HAIR KERATIN AMINO ACIDS 15 mg in 100 g NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 10 mg in 100 g BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 15 mg in 100 g ROSEMARY (UNII: IJ67X351P9) (ROSEMARY - UNII:IJ67X351P9) ROSEMARY 20 mg in 100 g Inactive Ingredients Ingredient Name Strength ARGAN OIL (UNII: 4V59G5UW9X) 10 mg in 100 g PEPPERMINT OIL TERPENELESS (UNII: 4CA0UO1N3Z) 10 mg in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-021-01 30 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 02/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/19/2024 Labeler - Shenzhen XiaoMai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen XiaoMai Manufacturing Co., Ltd. 712999147 manufacture(83872-021)