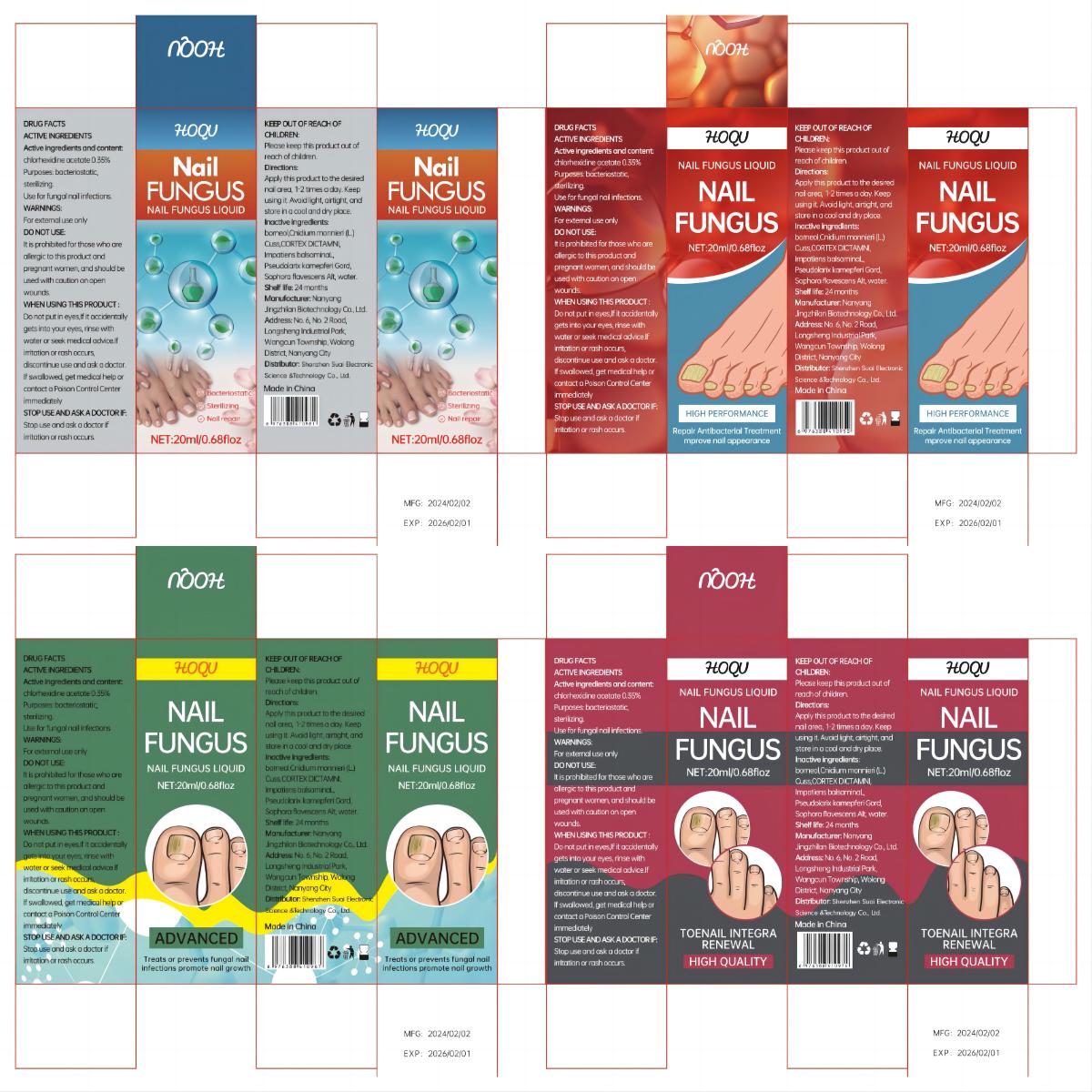
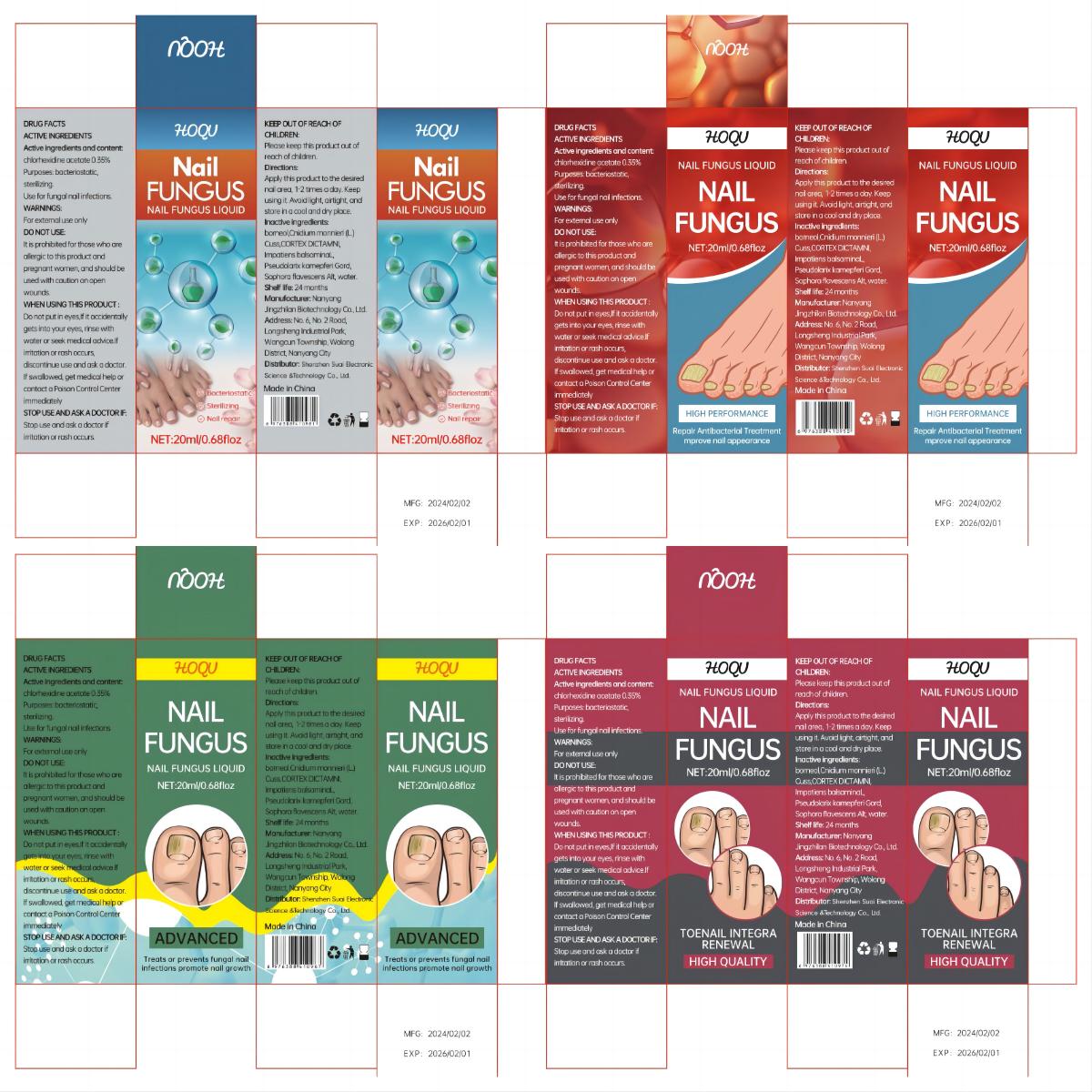
Label: HOQU NAIL FUNGUS LIQUID- nail fungus liquid liquid
- NDC Code(s): 84095-001-01
- Packager: Shenzhen Suai Electronic Science & Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HOQU NAIL FUNGUS LIQUID
nail fungus liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84095-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE ACETATE 0.35 g in 100 mL Inactive Ingredients Ingredient Name Strength IMPATIENS IRVINGII WHOLE (UNII: 2VRX978X4A) WATER (UNII: 059QF0KO0R) DICTAMNUS DASYCARPUS ROOT (UNII: 6153LEN214) BORNEOL (UNII: M89NIB437X) CNIDIUM OFFICINALE WHOLE (UNII: E9V83DB6BY) SOPHORA FLAVESCENS WHOLE (UNII: X8KX602M5L) HIBISCUS SYRIACUS BARK (UNII: U6PQI719P3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84095-001-01 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 02/18/2024 Labeler - Shenzhen Suai Electronic Science & Technology Co., Ltd. (444420123) Establishment Name Address ID/FEI Business Operations Shenzhen Suai Electronic Science & Technology Co., Ltd. 444420123 manufacture(84095-001) , label(84095-001)