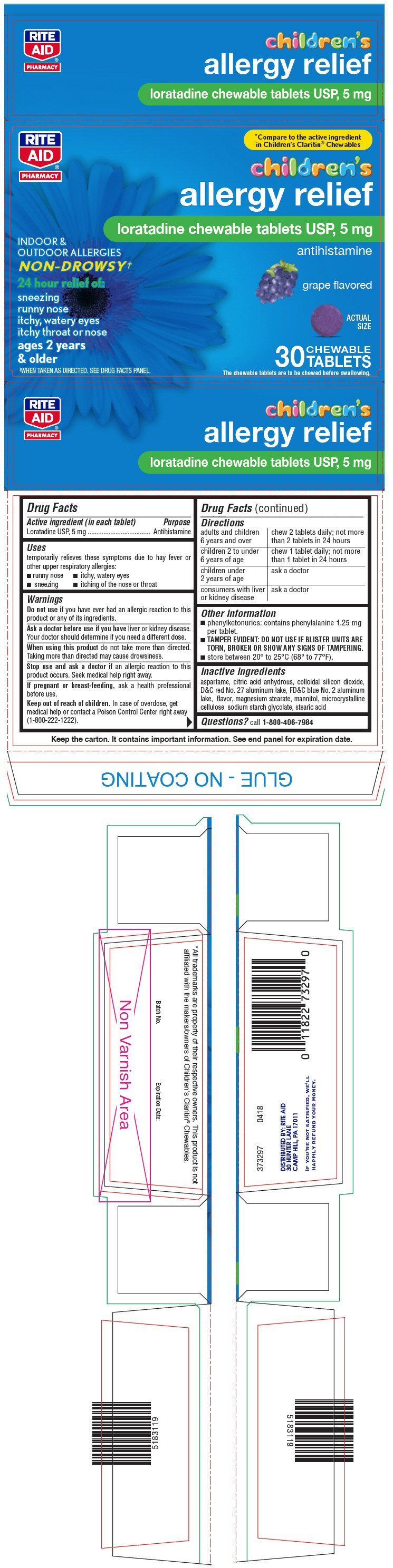
Label: LORATADINE tablet, chewable
- NDC Code(s): 11822-7240-0
- Packager: Rite Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 12, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each tablet)Loratadine USP, 5 mg
-
PurposeAntihistamine
-
Usestemporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - itchy, watery eyes - sneezing - itching of the nose or throat
-
WarningsDo not use if you have ever had an allergic reaction to this product or any of its ingredients. Ask a doctor before use if you have liver or kidney disease. Your doctor should determine ...
-
Directionsadults and children 6 years and overchew 2 tablets daily; not more than 2 tablets in 24 hours - children 2 to under 6 years of agechew 1 tablet daily; not more than 1 tablet in 24 ...
-
Other informationphenylketonurics: contains phenylalanine 1.25 mg per tablet. TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING. store between 20° to 25°C (68° to ...
-
Inactive ingredientsaspartame, citric acid anhydrous, colloidal silicon dioxide, D&C red No. 27 aluminum lake, FD&C blue No. 2 aluminum lake, flavor, magnesium stearate, mannitol, microcrystalline cellulose, sodium ...
-
Questions?call 1-800-406-7984
-
SPL UNCLASSIFIED SECTIONDISTRIBUTED BY: RITE AID - 30 HUNTER LANE - CAMP HILL, PA 17011
-
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack CartonRITE - AID® PHARMACY - *Compare to the active ingredient - in Children's Claritin® Chewables - children's - allergy relief - loratadine chewable tablets USP, 5 mg - INDOOR & OUTDOOR ALLERGIES ...
-
INGREDIENTS AND APPEARANCEProduct Information