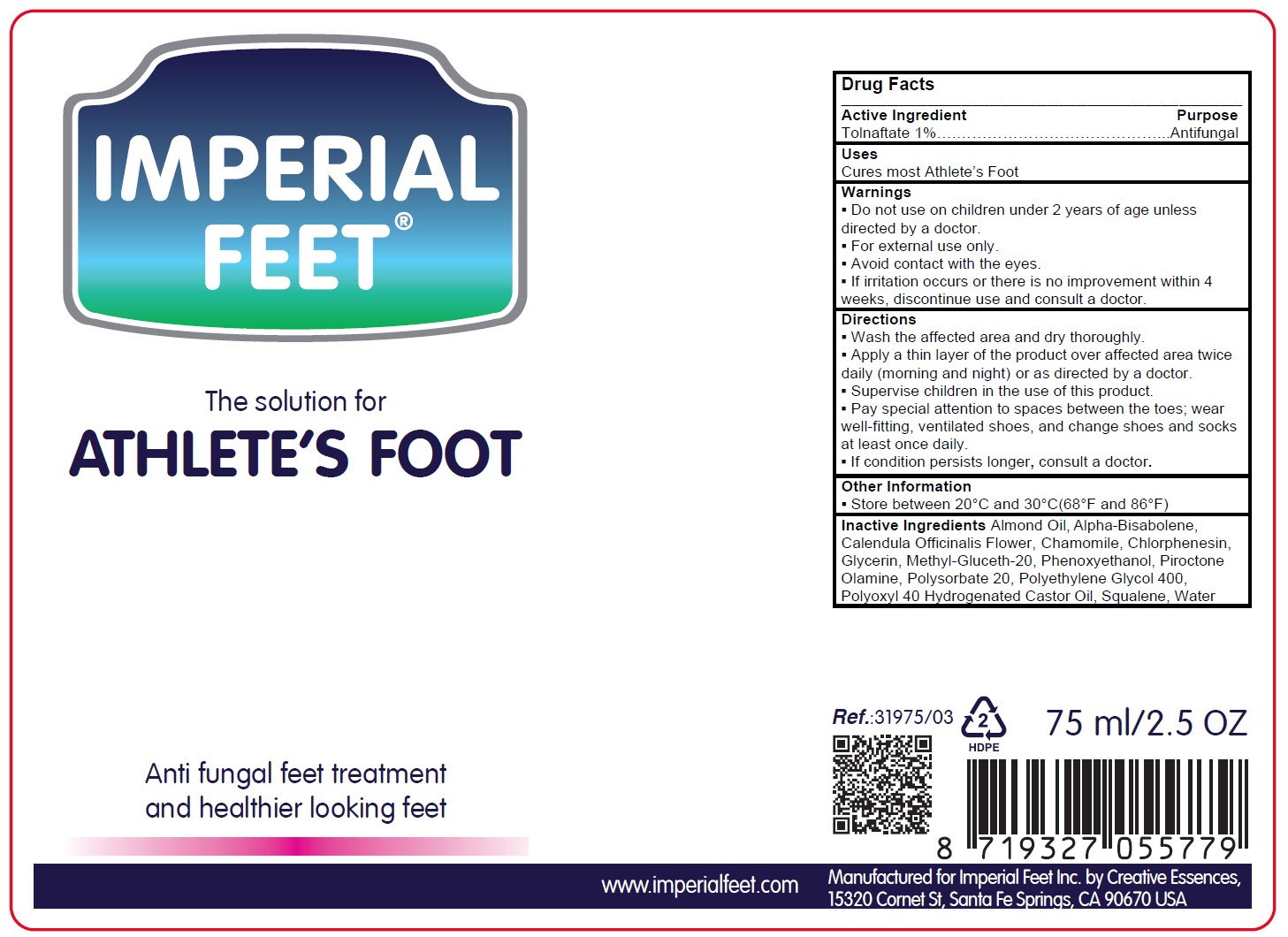
Label: ATHLETES FOOT- tolnaftate solution/ drops
- NDC Code(s): 83837-002-01
- Packager: Imperial Feet B.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Warnings
. Do not use on children under 2 years of age unless directed by a doctor.
. For external use only.
. Avoid contact with the eyes.
. If irritation occurs or there is no improvement within 4 weeks, discontinue use and consult a doctor. - Inactive Ingredient
- Other Information
- Purpose
-
Directions
- Wash the affected area and dry thoroughly.
- Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product.
- Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- If condition persists longer, consult a doctor.
- Use
- Principal Display Panel / Product Label
-
INGREDIENTS AND APPEARANCE
ATHLETES FOOT
tolnaftate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83837-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) 0.3 g in 100 mL CHLORPHENESIN (UNII: I670DAL4SZ) 0.2 g in 100 mL PIROCTONE OLAMINE (UNII: A4V5C6R9FB) 0.05 g in 100 mL ALMOND OIL (UNII: 66YXD4DKO9) 1 g in 100 mL POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) 13 g in 100 mL WATER (UNII: 059QF0KO0R) 0.3 g in 100 mL SQUALENE (UNII: 7QWM220FJH) 0.1 g in 100 mL METHYL GLUCETH-20 (UNII: J3QD0LD11P) 3 g in 100 mL .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) 0.1 g in 100 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.75 g in 100 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.1 g in 100 mL CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) 0.3 g in 100 mL POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 70 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 0.3 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83837-002-01 75 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 02/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 02/19/2024 Labeler - Imperial Feet B.V. (386790021) Registrant - Creative Essences Inc (079120182) Establishment Name Address ID/FEI Business Operations Creative Essences Inc 079120182 manufacture(83837-002)