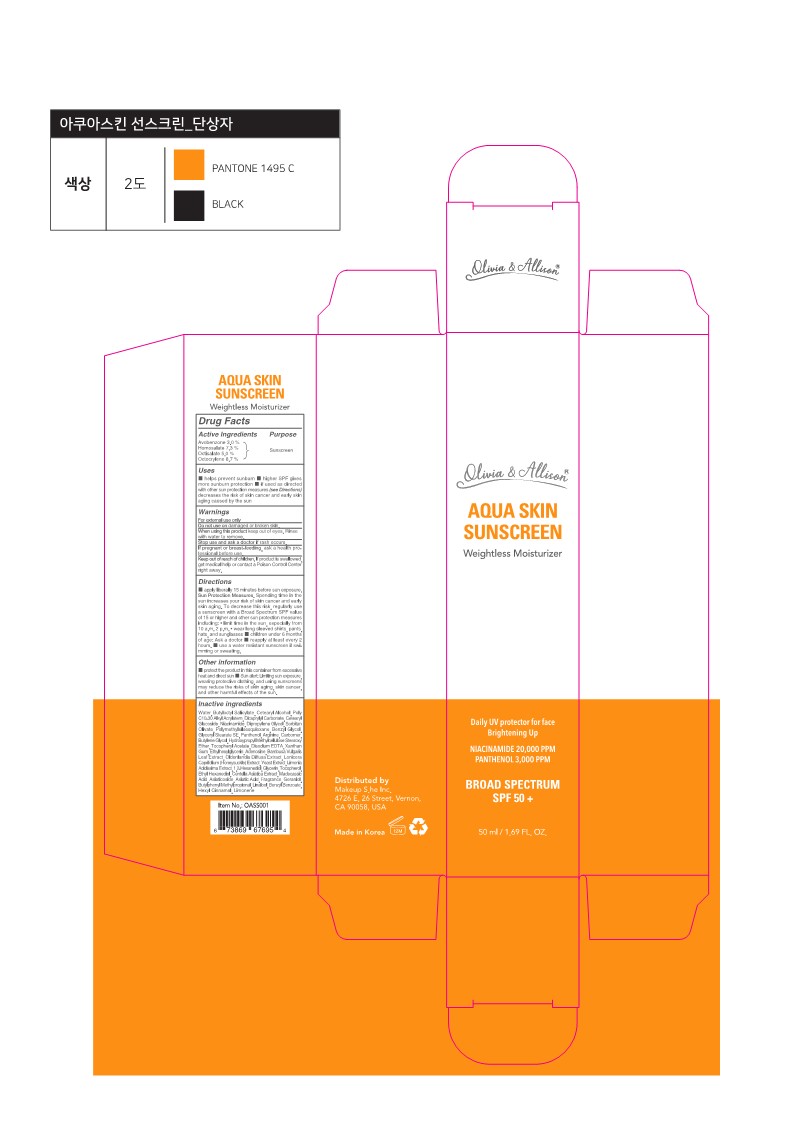
Label: OLIVIA AND ALLISON AQUA SKIN SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene cream
- NDC Code(s): 84122-050-01, 84122-050-02
- Packager: Makeup S.he, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use on
- When using the product
- Stop use and ask a doctor
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
■ apply liberally 15 minutes before sun exposure. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses ■ children under 6 months of age: Ask a doctor ■ reapply at least every 2 hours. ■ use a water-resistant sunscreen if swimming or sweating.
- Other information
-
Inactive ingredients
Water, Butyloctyl Salicylate, Cetearyl Alcohol, Poly C10-30 Alkyl Acrylate, Dicaprylyl Carbonate, Cetearyl Glucoside, Niacinamide, Dipropylene Glycol, Sorbitan Olivate, Polymethylsilsesquioxane, Benzyl Glycol, Glyceryl Stearate SE, Panthenol, Arginine, Carbomer, Butylene Glycol, Hydroxypropyl Methylcellulose Stearoxy Ether, Tocopheryl Acetate, Disodium EDTA, Xanthan Gum, Ethylhexylglycerin, Adenosine, Bambusa Vulgaris Leaf Extract, Oldenlandia Diffusa Extract, Lonicera Caprifolium (Honeysuckle) Extract, Yeast Extract, Limonia Acidissima Extract, 1,2-Hexanediol, Glycerin, Tocopherol, Ethyl Hexanediol, Centella Asiatica Extract, Madecassic Acid, Asiaticoside, Asiatic Acid, Fragrance, Geraniol, Butylphenyl Methylpropional, Linalool, Benzyl Benzoate, Hexyl Cinnamal, Limonene
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OLIVIA AND ALLISON AQUA SKIN SUNSCREEN
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84122-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2.5 g in 50 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.5 g in 50 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4.35 g in 50 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 3.65 g in 50 mL Inactive Ingredients Ingredient Name Strength DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) ETHYLENE GLYCOL MONOBENZYL ETHER (UNII: 06S8147L47) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ASIATICOSIDE (UNII: PKO39VY215) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) BENZYL BENZOATE (UNII: N863NB338G) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) ETHOHEXADIOL (UNII: M9JGK7U88V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) LIMONENE, (+)- (UNII: GFD7C86Q1W) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) XANTHAN GUM (UNII: TTV12P4NEE) GERANIOL (UNII: L837108USY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BAMBUSA VULGARIS LEAF (UNII: EMY54R518C) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) ASIATIC ACID (UNII: 9PA5A687X5) WATER (UNII: 059QF0KO0R) MADECASSIC ACID (UNII: M7O1N24J82) SCLEROMITRION DIFFUSUM (UNII: 291PPU5K9I) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ADENOSINE (UNII: K72T3FS567) YEAST (UNII: 3NY3SM6B8U) GLYCERIN (UNII: PDC6A3C0OX) TOCOPHEROL (UNII: R0ZB2556P8) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) NIACINAMIDE (UNII: 25X51I8RD4) DIPROPYLENE GLYCOL (UNII: E107L85C40) SORBITAN OLIVATE (UNII: MDL271E3GR) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PANTHENOL (UNII: WV9CM0O67Z) ARGININE (UNII: 94ZLA3W45F) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84122-050-02 1 in 1 BOX 02/26/2024 1 NDC:84122-050-01 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/26/2024 Labeler - Makeup S.he, Inc. (005613587) Establishment Name Address ID/FEI Business Operations Rebom Co., Ltd. 695951708 manufacture(84122-050)