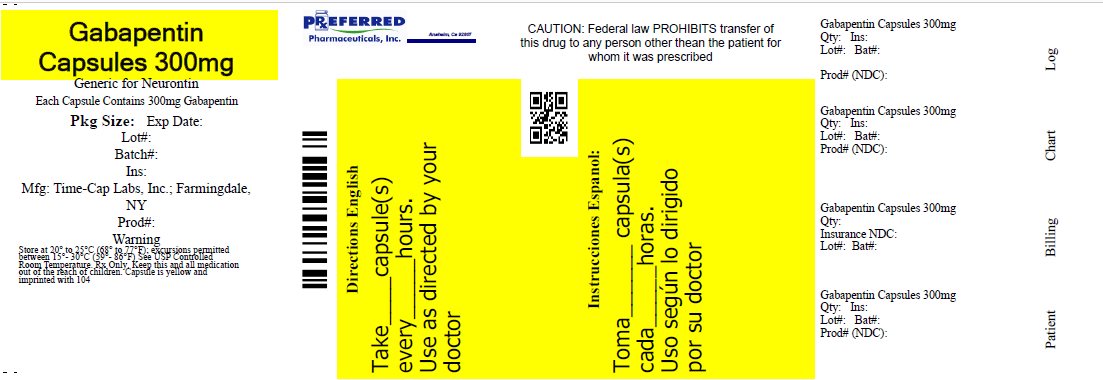
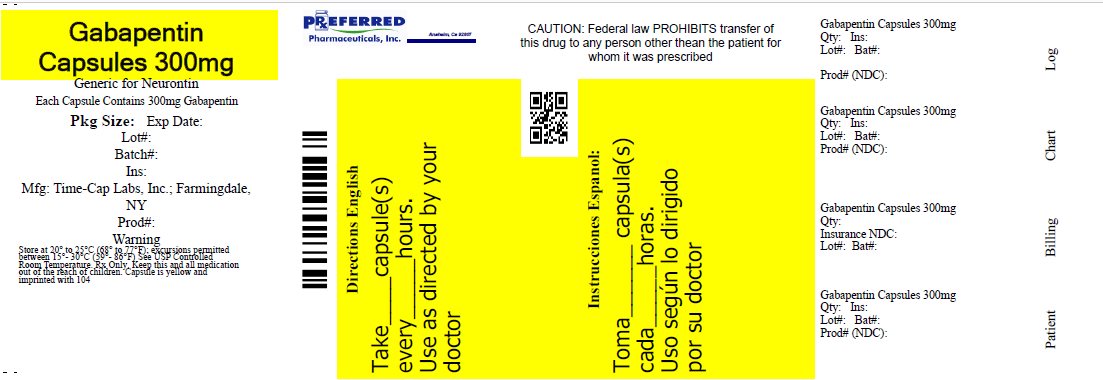
Label: GABAPENTIN capsule
-
NDC Code(s):
68788-7024-1,
68788-7024-2,
68788-7024-3,
68788-7024-4, view more68788-7024-6, 68788-7024-8, 68788-7024-9
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 49483-606
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 7, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
MEDICATION GUIDE
Gabapentin (gab'' a pen' tin)Capsules
What is the most important information I should know about gabapentin capsules?
Do not stop taking gabapentin capsules without first talking to your healthcare provider.
Stopping gabapentin capsules suddenly can cause serious problems.
Gabapentin capsules can cause serious side effects including:
1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin capsules may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
· thoughts about suicide or dying
· attempts to commit suicide
· new or worse depression
· new or worse anxiety
· feeling agitated or restless
· panic attacks
· trouble sleeping (insomnia)
· new or worse irritability
· acting aggressive, being angry, or violent
· acting on dangerous impulses
· an extreme increase in activity and talking (mania)
· other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
· Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
· Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop taking gabapentin capsules without first talking to a healthcare provider.
· Stopping gabapentin capsules suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
· Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
2. Changes in behavior and thinking -Using gabapentin capsules in children 3 to 12 years of age can cause emotional changes, aggressive behavior, problems with concentration, restlessness, changes in school performance, and hyperactivity.
3. Gabapentin capsules may cause a serious or life-threatening allergic reaction that may affect your skin or other parts of your body such as your liver or blood cells. This may cause you to be hospitalized or to stop gabapentin capsule. You may or may not have a rash with an allergic reaction caused by gabapentin capsule. Call a healthcare provider right away if you have any of
The following symptoms:
· skin rash
· hives
· difficulty breathing
· fever
· swollen glands that do not go away
· swelling of your face, lips, throat or tongue
· yellowing of your skin or of the whites of the eyes
· unusual bruising or bleeding
· severe fatigue or weakness
· unexpected muscle pain
· frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking gabapentin capsules.
What are gabapentin capsules?
Gabapentin capsules are a prescription medicine used to treat:
· Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults.
· Partial seizures when taken together with other medicines in adults and children 3 years of age and older with seizures.
Who should not take gabapentin capsules?
Do not take gabapentin capsules if you are allergic to gabapentin or any of the other ingredients in gabapentin capsules. See the end of this Medication Guide for a complete list of ingredients in gabapentin capsules.
What should I tell my healthcare provider before taking gabapentin capsules?
Before taking gabapentin capsules, tell your healthcare provider if you:
· have or have had kidney problems or are on hemodialysis
· have or have had depression, mood problems, or suicidal thoughts or behavior
· have diabetes
· are pregnant or plan to become pregnant. It is not known if gabapentin capsules can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking gabapentin capsules. You and your healthcare provider will decide if you should take gabapentin capsules while you are pregnant.
o Pregnancy Registry: If you become pregnant while taking gabapentin capsules, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
· are breast-feeding or plan to breast-feed. Gabapentin capsules can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take gabapentin capsules.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking gabapentin capsules with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take gabapentin capsules?
· Take gabapentin capsules exactly as prescribed. Your healthcare provider will tell you how much gabapentin capsules to take.
o Do not change your dose of gabapentin capsules without talking to your healthcare provider.
o Take gabapentin capsules with water.
· Gabapentin capsules can be taken with or without food. If you take an antacid containing aluminum and magnesium, such as Maalox®, Mylanta®, Gelusil®, Gaviscon® , or Di-Gel®, you should wait at least 2 hours before taking your next dose of gabapentin capsules.
If you take too much gabapentin capsules, call your healthcare provider or your local Poison Control Center right away at 1-800-222-1222.
What should I avoid while taking gabapentin capsules?
· Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking gabapentin capsules without first talking with your healthcare provider. Taking gabapentin capsules with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
· Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin capsules affects you. Gabapentin capsules can slow your thinking and motor skills.
What are the possible side effects of gabapentin capsules?
Gabapentin capsules may cause serious side effects including:
See “What is the most important information I should know about gabapentin capsules?”
· problems driving while using gabapentin capsules. See “What I should avoid while taking gabapentin capsules?”
· sleepiness and dizziness which could increase the occurrence of accidental injury, including falls
· The most common side effects of gabapentin capsules include:
· lack of coordination • feeling tired
· viral infection • fever
· feeling drowsy • jerky movements
· nausea and vomiting • difficulty with coordination
· difficulty with speaking • double vision
· tremor • unusual eye movement
· swelling, usually of legs and feet
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of gabapentin capsules. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to Time Cap Laboratories 1 877 290-4008 and/or FDA at 1-800-FDA-1088.
How should I store gabapentin capsules?
· Store gabapentin capsules between 68°F to 77°F (20°C to 25°C).
Keep gabapentin capsules and all medicines out of the reach of children.
General information about the safe and effective use of gabapentin capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use gabapentin capsules for a condition for which it was not prescribed. Do not give gabapentin capsules to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about gabapentin capsules. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about gabapentin capsules that was written for healthcare professionals.
What are the ingredients in gabapentin capsules?
Active ingredient: gabapentin, USP
Inactive ingredients in the capsules: lactose monohydrate, corn starch, and talc.
The 100 mg capsule shell contains gelatin and titanium dioxide.
The 300 mg capsule shell contains gelatin, titanium dioxide, and yellow iron oxide.
The 400 mg capsule shell contains gelatin, red iron oxide, titanium dioxide, and yellow iron oxide. The imprinting ink contains FD&C Blue No. 2, propylene glycol and shellac.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Rx only
Manufactured for:
Time-Cap-Labs, INC,
7 Michael Avenue, Farmingdale, NY 11735, USA
Manufactured by:
Marksans Pharma Ltd.
Plot No. L-82, L-83, Verna Indl. Estate,
Verna, Goa-403 722, India
Rev. 11/17
Repackaged By: Preferred Pharmaceuticals Inc.
- HOW SUPPLIED
- 300mg Gabapentin Capsule Package Label
-
INGREDIENTS AND APPEARANCE
GABAPENTIN
gabapentin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68788-7024(NDC:49483-606) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 300 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color yellow Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 104 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-7024-2 20 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 2 NDC:68788-7024-4 28 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 3 NDC:68788-7024-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 4 NDC:68788-7024-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 5 NDC:68788-7024-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 6 NDC:68788-7024-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 7 NDC:68788-7024-8 120 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090007 06/01/2020 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 REPACK(68788-7024)