Label: WELLY FIRST AID KIT- hydrocortisone, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, ibuprofen kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 72663-152-05 - Packager: Welly Health PBC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
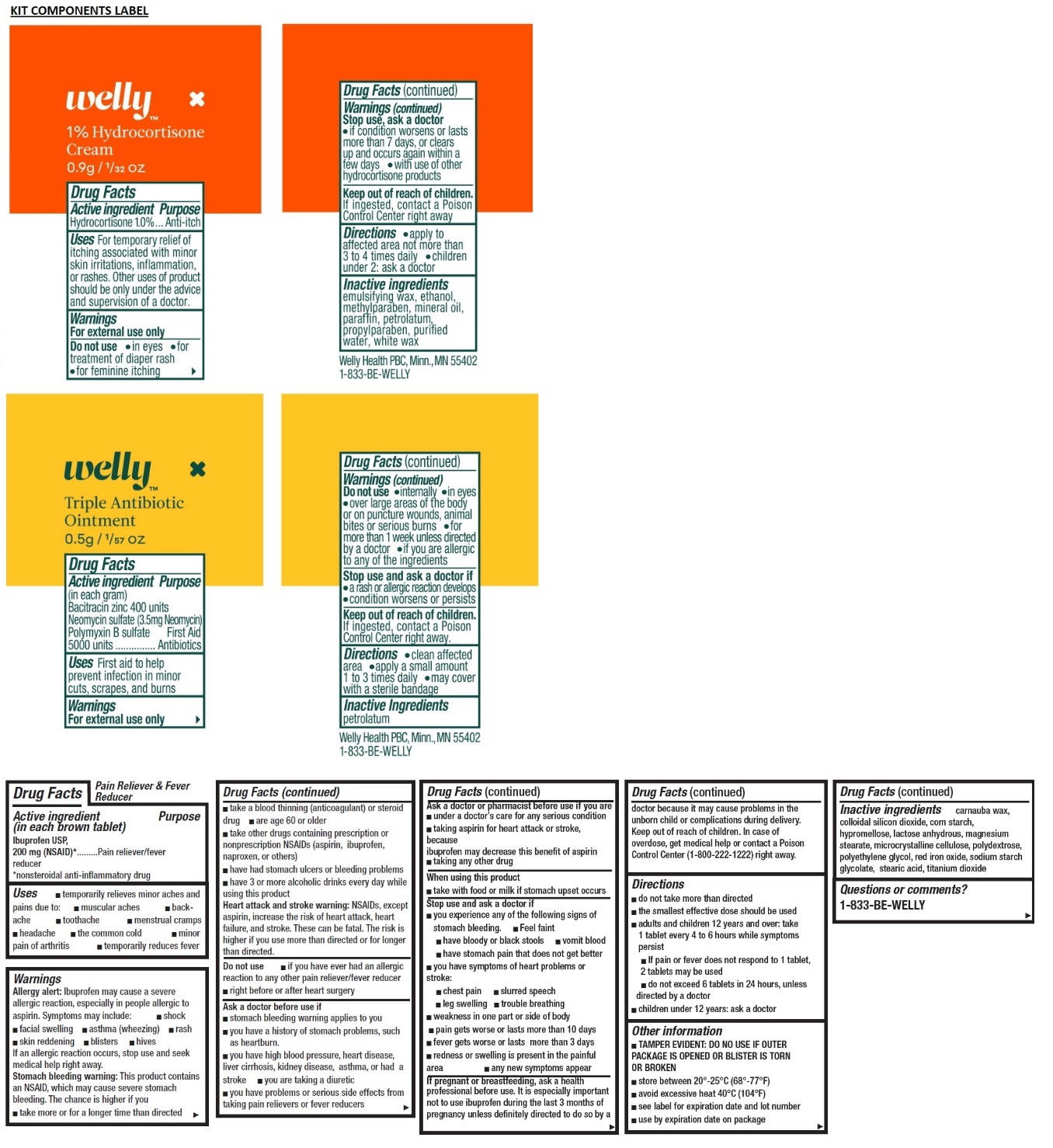
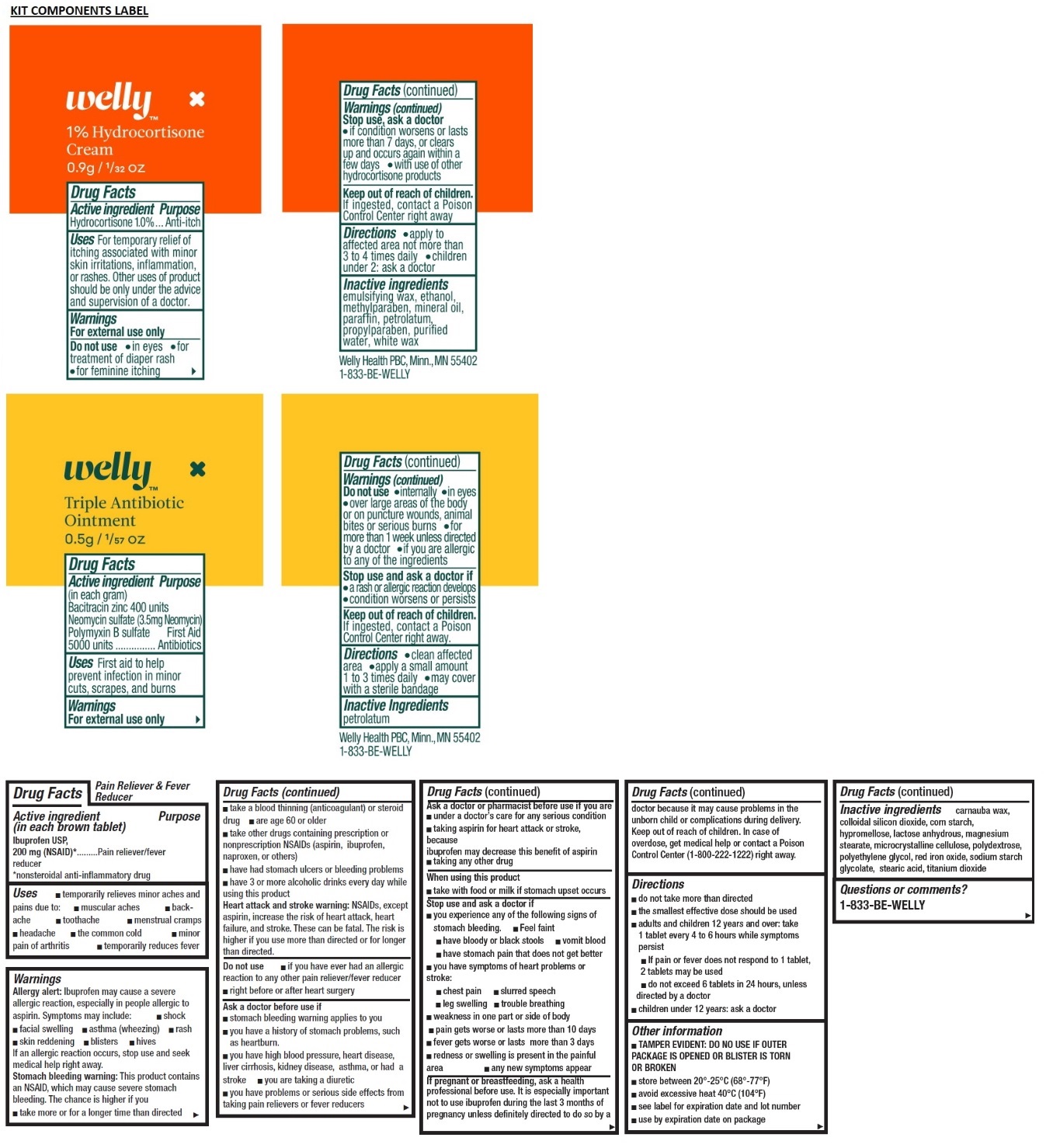
- 1% Hydrocortisone Cream
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Triple Antibiotic Ointment
- Active Ingredient (in each gram)
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- internally
- in eyes
- over large areas of the body or on puncture wounds, animal bites or serious burns
- for more than 1 week unless directed by a doctor
- if you are allergic to any of the ingredients
Stop use and ask a doctor if
- a rash or allergic reactions develops
- condition worsens or persists
- Directions
- Inactive Ingredients
- Pain Relief and Fever Reducer
- Active ingredient (in each brown tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- shock
- facial swelling
- asthma (wheezing)
- rash
- skin reddening
- blisters
- hives
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- take more or for a longer time than directed
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have had stomach ulcers or bleeding problems
- have 3 or more alcoholic drinks every day while using this product
Heart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn.
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
Ask a doctor or pharmacist before use if you are
- under a doctor’s care for any serious condition
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding.
- Feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- slurred speech
- leg swelling
- trouble breathing
- weakness in one part or side of body
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breastfeeding,
ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery. -
Directions
- do not take more than directed
- the smallest effective dose should be used
- adults and children 12 years and over: take 1 tablet every 4 to 6 hours while symptoms persist
- If pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
- children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
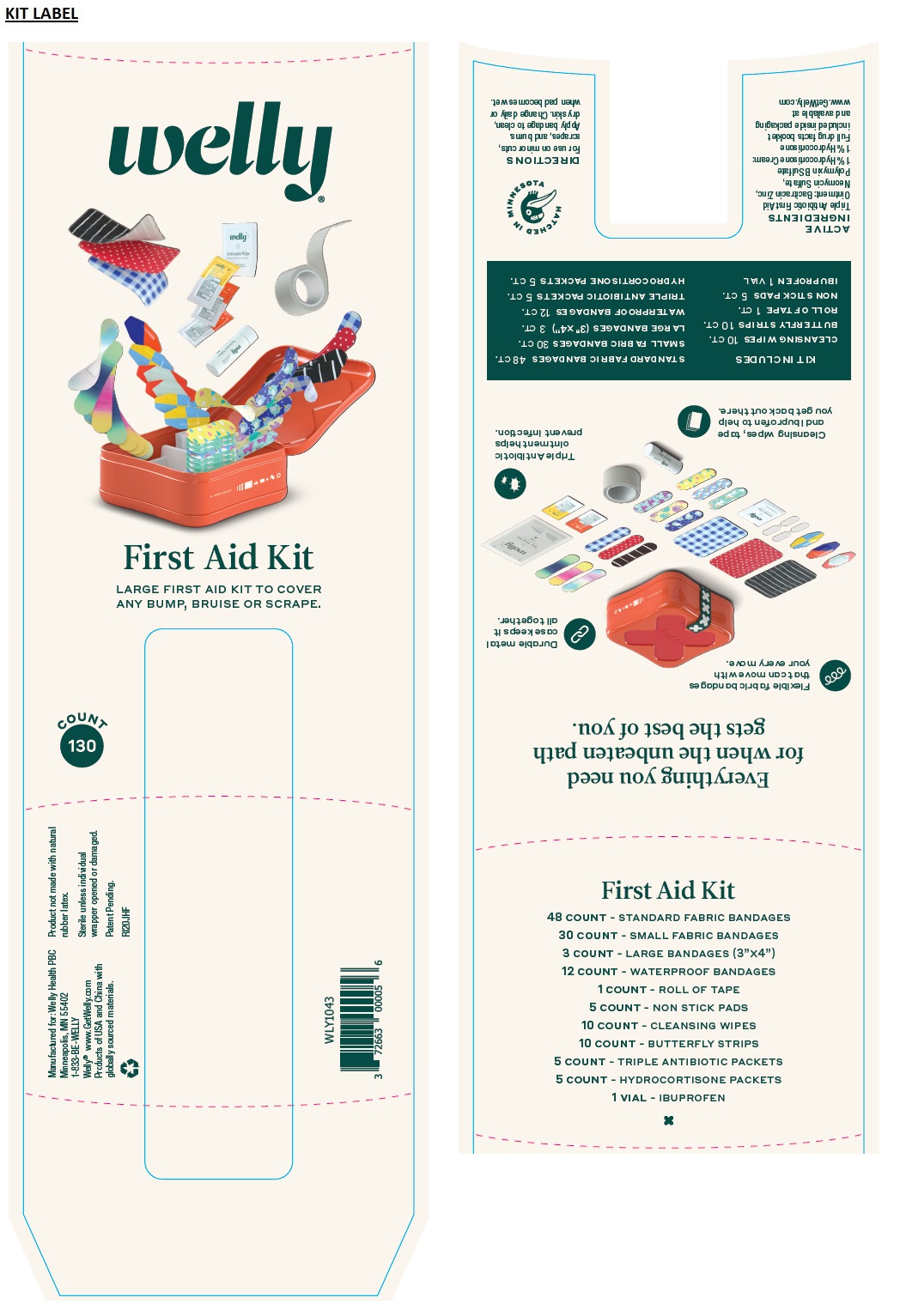
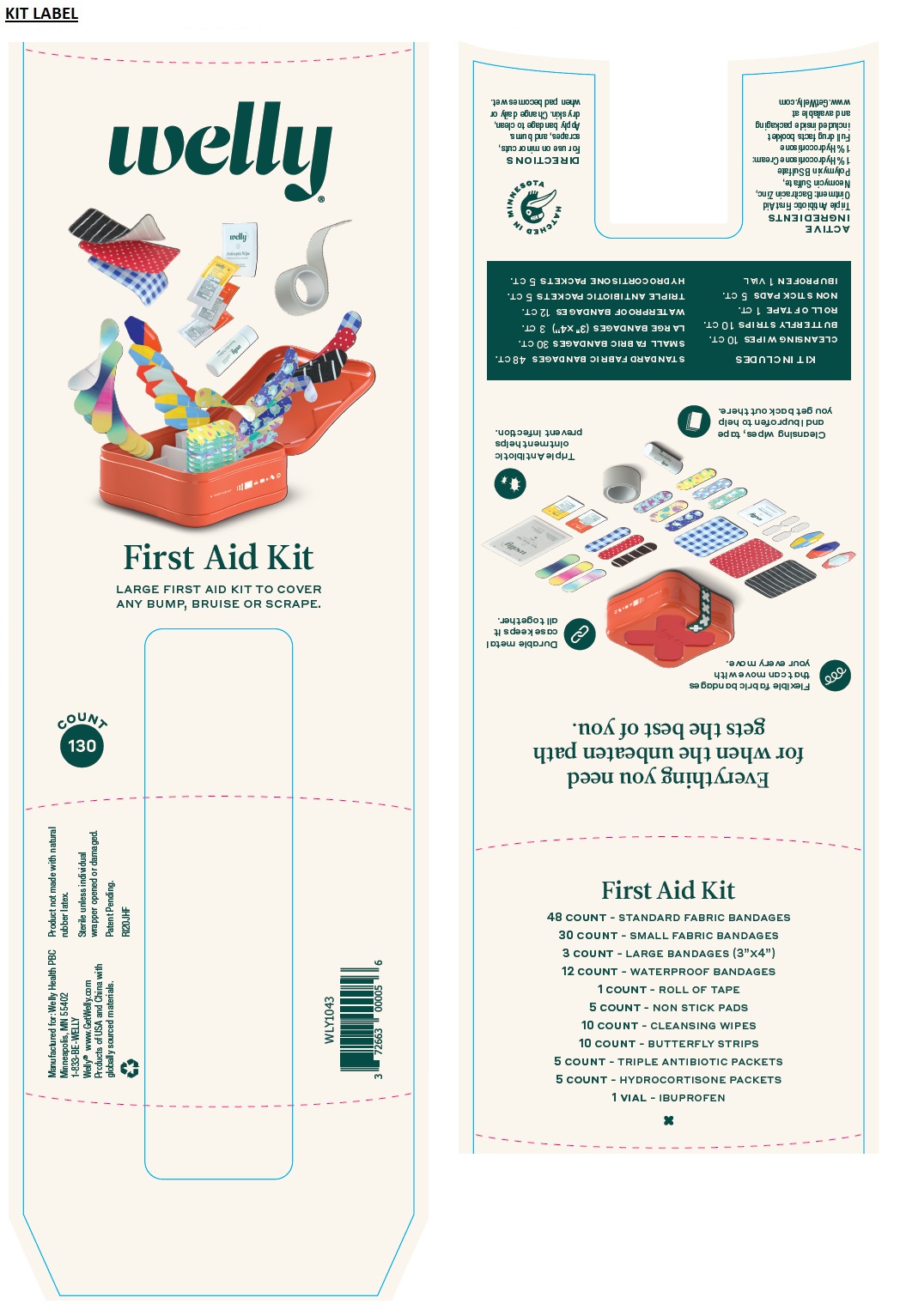
First Aid Kit
LARGE FIRST AID KIT TO COVER ANY BUMP, BRUISE OR SCRAPE.
Everything you need for when the unbeaten path gets the best of you.
Flexible fabric bandages that can move with your every move.
Durable metal case keeps it all together.
Cleansing wipes, tape and ibuprofen to help you get back out there.
Triple Antibiotic ointment helps prevent infection.
KIT INCLUDES
48 COUNT - STANDARD FABRIC BANDAGES
30 COUNT - SMALL FABRIC BANDAGES
3 COUNT - LARGE BANDAGES (3"x4")
12 COUNT - WATERPROOF BANDAGES
1 COUNT - ROLL OF TAPE
5 COUNT - NON STICK PADS
10 COUNT - CLEANSING WIPES
10 COUNT - BUTTERFLY STRIPS
5 COUNT - TRIPLE ANTIBIOTIC PACKETS
5 COUNT - HYDROCORTISONE PACKETS
1 VIAL - IBUPROFEN
Manufactured for: Welly Health PBC
Minneapolis, MN 55402
1-833-BE-WELLYRecycle me!
Welly TM www.GetWelly.com
Products of USA and China with globally sourced materials
Product not made with natural rubber latex
Sterile unless individual wrapper opened or damaged.
Patent Pending
- Packaging
-
INGREDIENTS AND APPEARANCE
WELLY FIRST AID KIT
hydrocortisone, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, ibuprofen kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72663-152 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-152-05 1 in 1 KIT; Type 0: Not a Combination Product 04/06/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 POUCH 4.5 mL Part 2 5 POUCH 2.5 mL Part 3 1 BOTTLE 16 Part 4 10 POUCH 10 Part 1 of 4 ANTI-ITCH
hydrocortisone creamProduct Information Item Code (Source) NDC:72663-580 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) WHITE WAX (UNII: 7G1J5DA97F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.9 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/25/2019 Part 2 of 4 ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:72663-560 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 6 mg in 1 mL NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 3.5 mg in 1 mL POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 0.77 mg in 1 mL Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/25/2019 Part 3 of 4 PAIN RELIEF AND FEVER REDUCER
ibuprofen tabletProduct Information Item Code (Source) NDC:72663-428 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code 44291 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 16 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/06/2020 Part 4 of 4 WELLY CLEANSING WIPE
cleansing (cold creams, cleansing lotions, liquids, and pads)Product Information Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/06/2020 Labeler - Welly Health PBC (116766884)