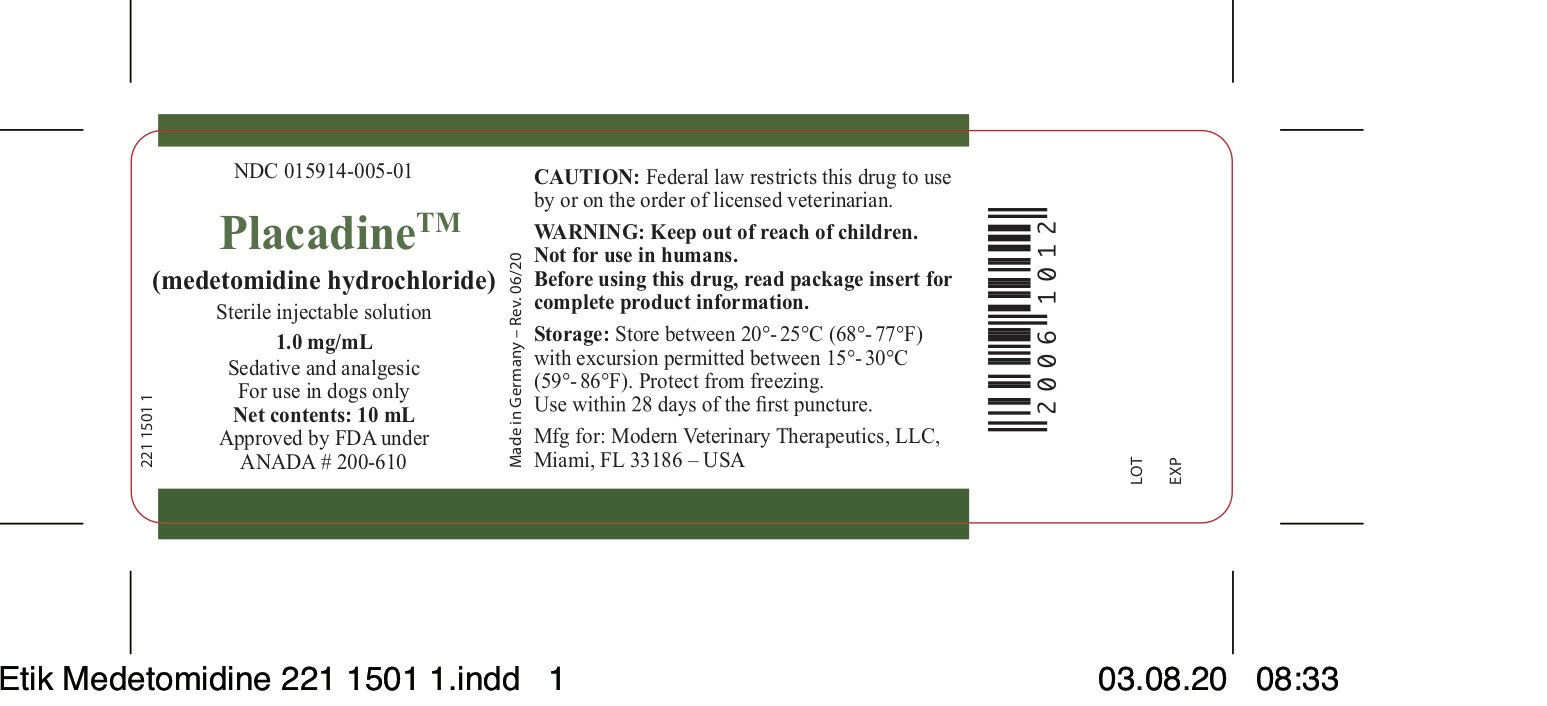
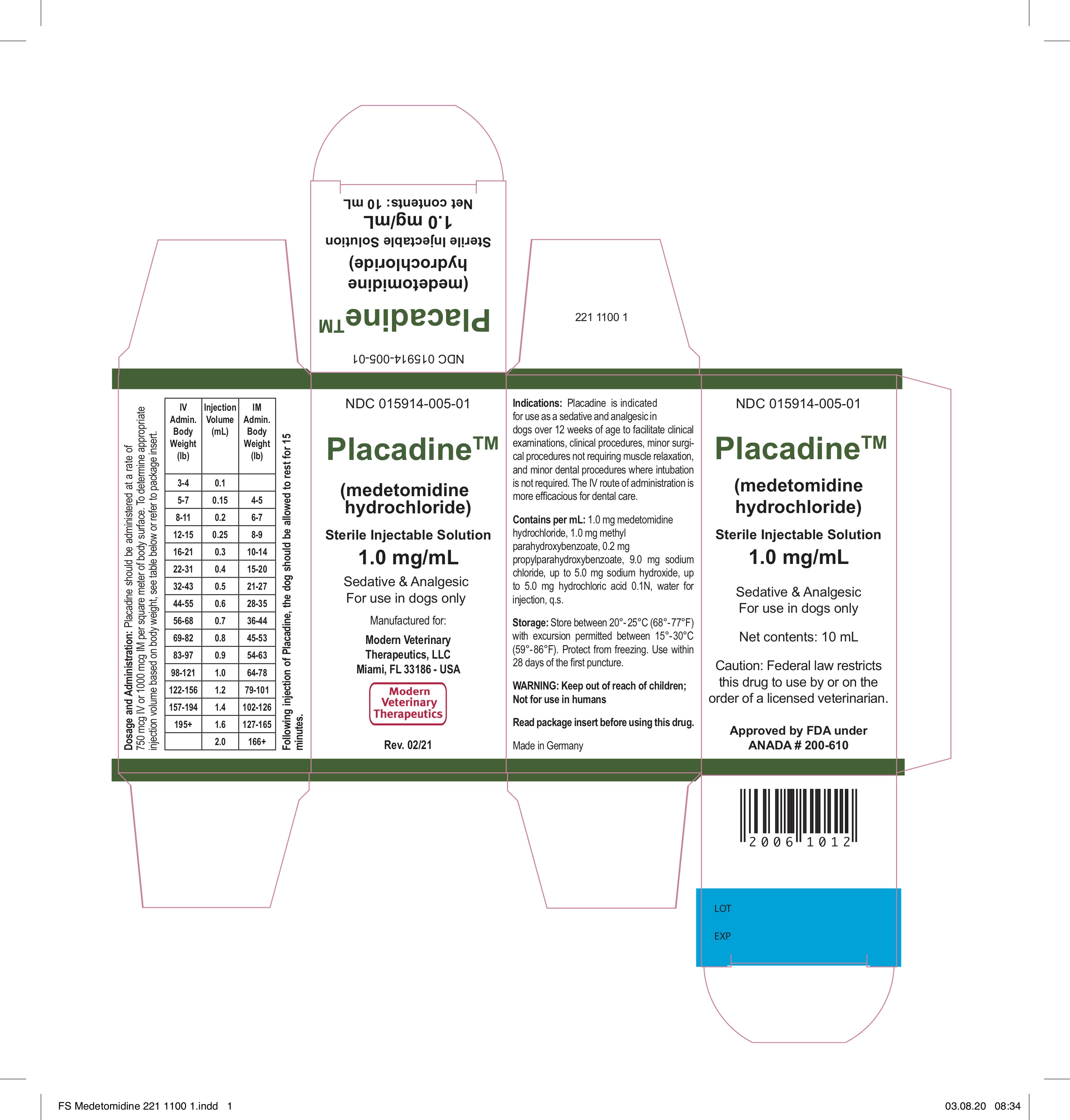
Label: PLACADINE- medetomidine hydrochloride injection, solution
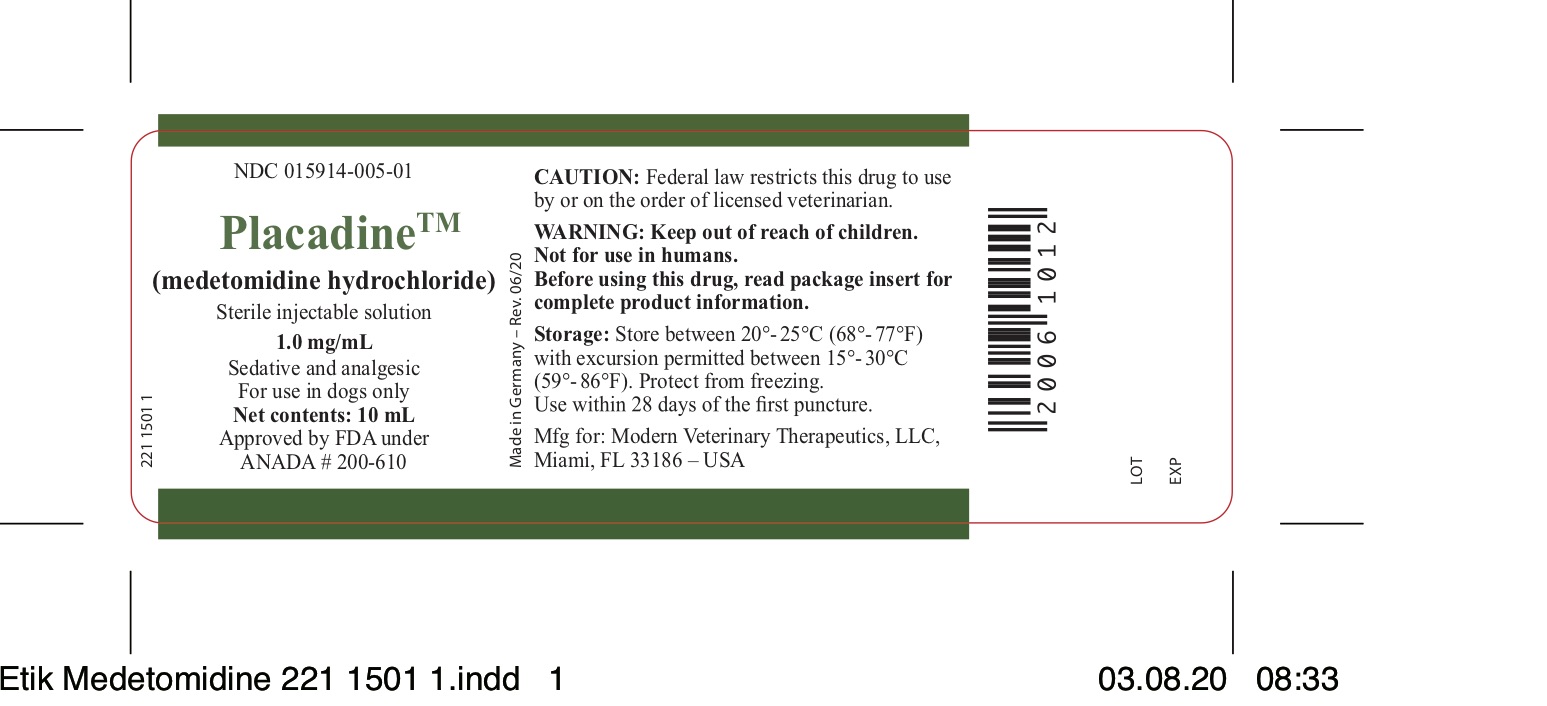
- NDC Code(s): 15914-005-01
- Packager: Modern Veterinary Therapeutics LLC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
Drug Label Information
Updated December 9, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
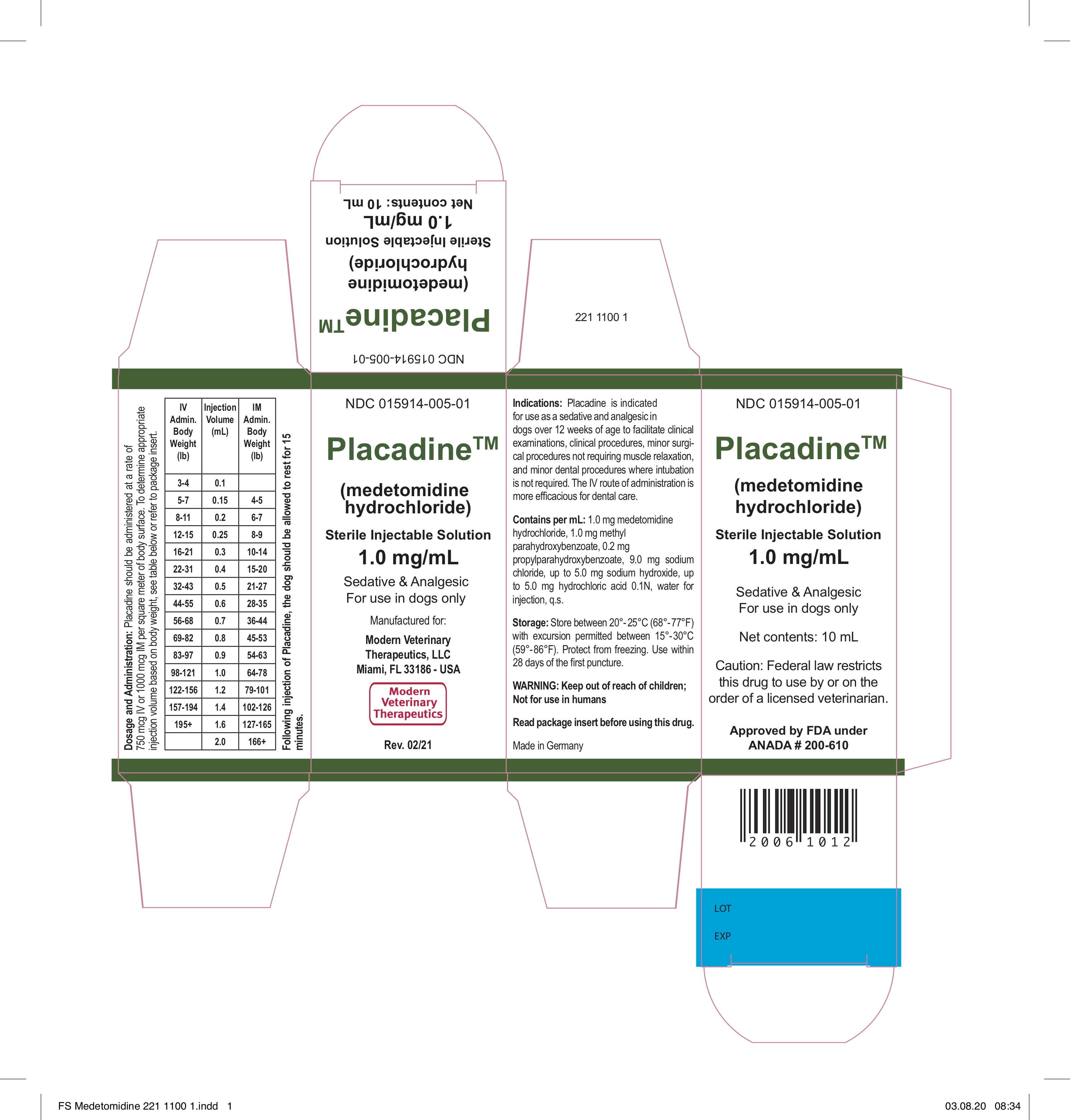
- Placadine™(medetomidine hydrochloride)
- CAUTION
-
DESCRIPTION
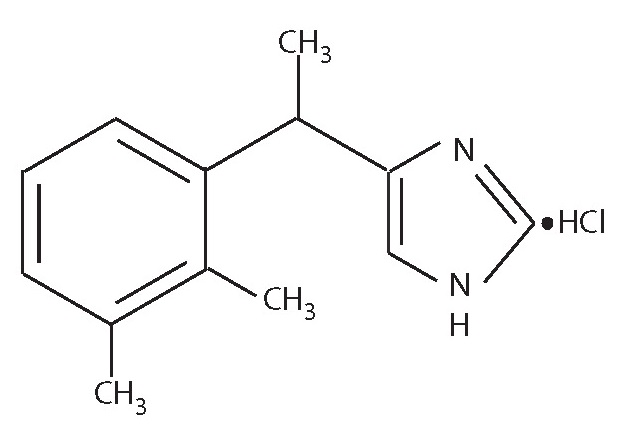
Placadine (medetomidine hydrochloride) is a synthetic α2-adrenoreceptor agonist with sedative and analgesic properties. The chemical name is (±)-4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole monohydrochloride. It is a white, or almost white, crystalline, water soluble substance having a molecular weight of 236.7. The molecular formula is C13H16N2•HCl and the structural formula is:
-
CLINICAL PHARMACOLOGY
Medetomidine is a potent non-narcotic α2-adrenoreceptor agonist which produces sedation and analgesia. These effects are dose dependent in depth and duration. Profound sedation and recumbency, with reduced sensitivity to environmental stimuli (sounds, etc.), are seen with medetomidine.
The pharmacological restraint and pain relief provided by medetomidine facilitates handling dogs and aids in the conduct of diagnostic or therapeutic procedures. It also facilitates minor surgical procedures (with or without local anesthesia) and dental care where intubation is not required. Spontaneous muscle contractions (twitching) can be expected in some dogs sedated with medetomidine.
With medetomidine administration, blood pressure is initially increased due to peripheral vasoconstriction and thereafter drops to normal or slightly below normal levels. The initial vasopressor response is accompanied by a compensatory marked decrease in heart rate mediated by a vagal baroreceptor mechanism. The bradycardia may be partially prevented by prior (at least 5 minutes before) intravenous administration of an anticholinergic agent (see PRECAUTIONS). A transient change in the conductivity of the cardiac muscle may occur, as evidenced by atrioventricular blocks. Cardiovascular changes (such as profound bradycardia and second degree heart block) equally affect both heartworm negative and asymptomatic heartworm positive dogs.
Respiratory responses include an initial slowing of respiration within a few seconds to 1–2 minutes after administration, increasing to normal within 120 minutes. An initial decrease in tidal volume is followed by an increase. When medetomidine was given at 3 and 5 times the recommended dose IV, and 5 and 10 times IM, effects were not intensified but were prolonged.
-
INDICATIONS
Placadine is indicated for use as a sedative and analgesic in dogs over 12 weeks of age to facilitate clinical examinations, clinical procedures, minor surgical procedures not requiring muscle relaxation, and minor dental procedures where intubation is not required. The IV route of administration is more efficacious for dental care.
-
WARNINGS
Keep out of reach of children. Not for use in humans.
Medetomidine hydrochloride can be absorbed and may cause irritation following direct exposure to skin, eyes, or mouth. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If irritation or other adverse reaction occurs (e.g., sedation, hypotension, bradycardia), seek medical attention. In case of accidental oral exposure or injection, seek medical attention. Precaution should be used while handling and using filled syringes.
Users with cardiovascular disease (e.g., hypertension or ischemic heart disease) should take special precautions to avoid any exposure to this product.
The safety data sheet (SDS) contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance or to obtain a copy of the SDS, contact Modern Veterinary Therapeutics, LLC at 407-852-8039 or www.modernveterinarytherapeutics.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae
- NOTE TO PHYSICIANS
- CONTRAINDICATIONS
-
PRECAUTIONS
In extremely nervous or excited dogs, levels of endogenous catecholamines are high due to the animal’s state of agitation. The pharmacological response elicited by α2-agonists (e.g., medetomidine) in such animals is often reduced, with depth and duration of sedative/analgesic effects ranging from slightly diminished to nonexistent. Highly agitated dogs should therefore be put at ease and allowed to rest quietly prior to receiving Placadine. Allowing dogs to rest quietly for 10 to 15 minutes after injection may improve the response to Placadine.
In dogs not responding satisfactorily to treatment with Placadine, repeat dosing is not recommended. Caution should be exercised when handling sedated animals. Handling or any other sudden stimuli may cause a defense reaction in an animal that is sedated.
Placadine is a potent α2-agonist which should be used with caution with other sedative or analgesic drugs. Additive or synergistic effects are likely, possibly resulting in overdose. Although bradycardia may be partially prevented by prior (at least 5 minutes before) intravenous administration of an anticholinergic agent, the administration of anticholinergic agents to treat bradycardia either simultaneously with medetomidine or following sedation with medetomidine could lead to adverse cardiovascular effects.
Special care is recommended when treating very young animals and older animals. Information on the possible reproductive effects of medetomidine is limited; therefore, the drug is not recommended for use in dogs used for breeding purposes or in pregnant dogs.
-
ADVERSE REACTIONS
As with all α2-agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation), exists. Incidents of prolonged sedation, bradycardia, cyanosis, vomiting, apnea, death from circulatory failure with severe congestion of lungs, liver, kidney and recurrence of sedation after initial recovery have been reported.
-
SIDE EFFECTS
Bradycardia with occasional atrioventricular blocks will occur together with decreased respiratory rates. Body temperature is slightly or moderately decreased. Urination typically occurs during recovery at about 90 to 120 minutes posttreatment. In approximately 10% of treated dogs, occasional episodes of vomiting occur between 5 to 15 minutes posttreatment. An increase in blood glucose concentration is seen due to α2-adrenoreceptor-mediated inhibition of insulin secretion.
-
ANIMAL SAFETY
In target animal safety studies, medetomidine was tolerated in dogs at up to 5 times the recommended IV dose and up to 10 times the recommended IM dose. A single IV administration of 10 times the recommended dose in dogs caused a prolonged anesthesia-like condition accompanied by an increased level of spontaneous muscle contractions (twitching). Repeated IV doses of 3 or 5 times the recommended dose caused a profound sedation, bradycardia, and reduced respiratory rates over several hours, accompanied in some animals by occasional spontaneous twitching. Death (approximately 1 in 40,000 treatments) has been noted in clinical use with doses at 2 times the recommended dose of medetomidine hydrochloride.
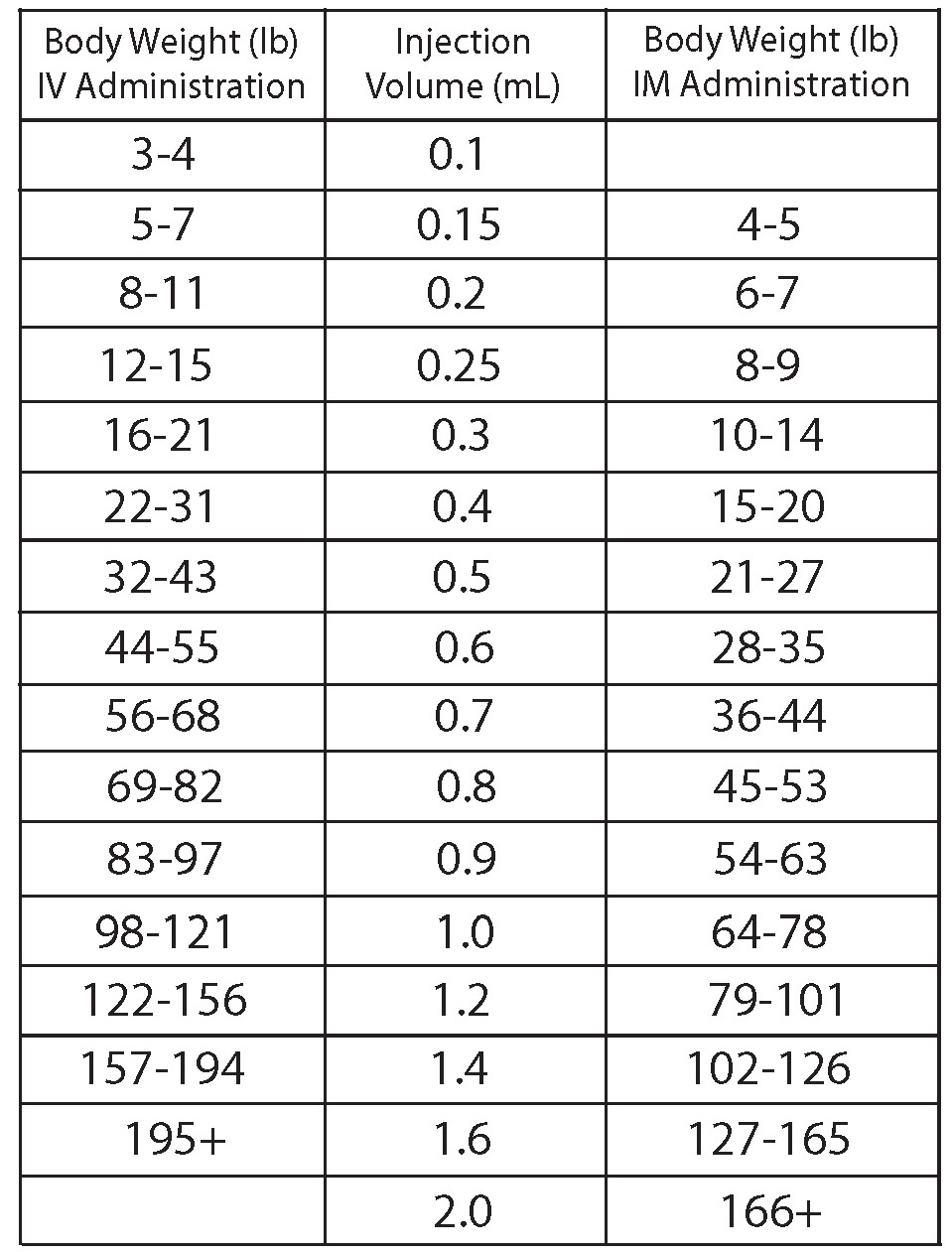
- DOSAGE AND ADMINISTRATION
- STORAGE
- HOW SUPPLIED
- Manufactured for: Modern Veterinary Therapeutics, LLC Miami, Florida 33186 - USAMade in GermanyRev. # 02.21
- BOX and VIAL
-
INGREDIENTS AND APPEARANCE
PLACADINE
medetomidine hydrochloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:15914-005 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEDETOMIDINE HYDROCHLORIDE (UNII: BH210P244U) (MEDETOMIDINE - UNII:MR15E85MQM) MEDETOMIDINE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) 1 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.2 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.0 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) 5.0 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) 5.0 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15914-005-01 1 in 1 BOX 1 10 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200610 02/28/2019 Labeler - Modern Veterinary Therapeutics LLC (197157139) Registrant - Modern Veterinary Therapeutics LLC (197157139)