Label: SMART NUMB LIDOCAINE CREAM- lidocaine cream
- NDC Code(s): 76092-201-02
- Packager: M.J. Winston International, Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
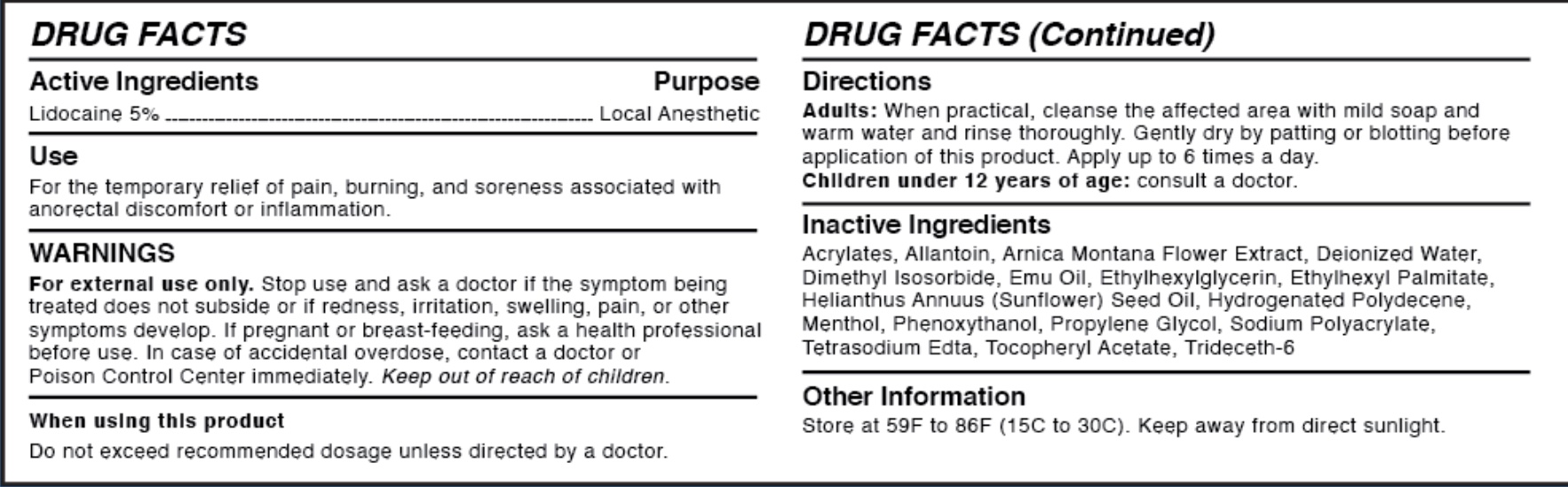
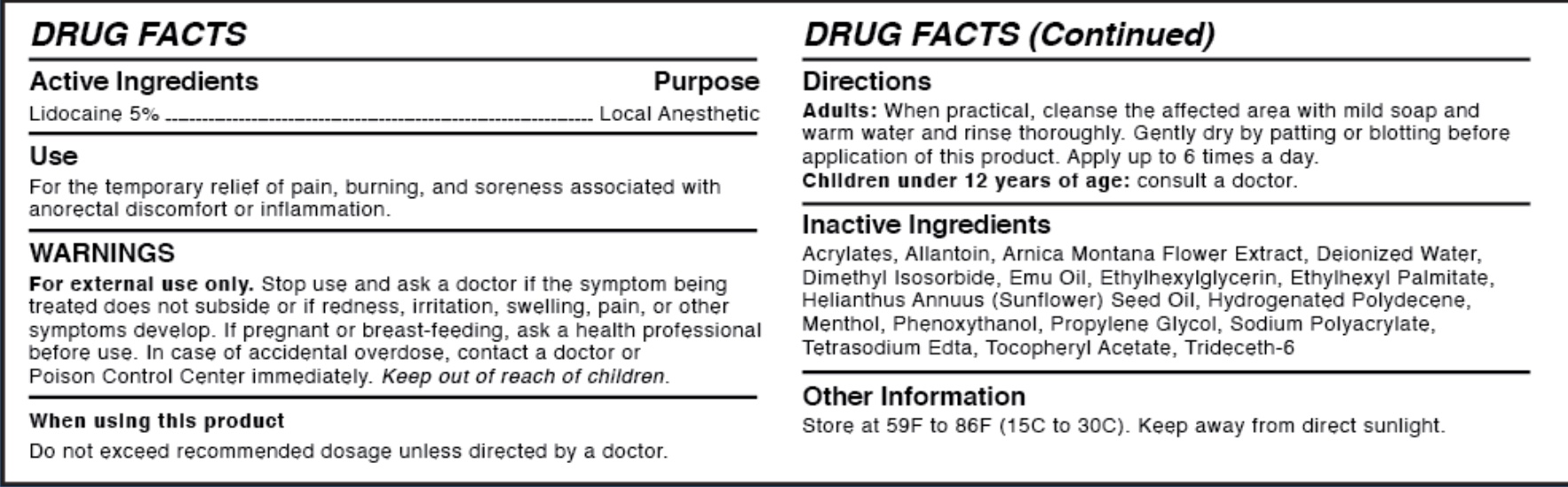
WARNINGS
WARNINGS
For external use only.
Stop use and ask a doctor if the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop. If pregnant or breast-feeding, ask a health professional before use.
In case of accidental overdose, contact a doctor or Poison Control Center immediately. Keep out of reach of children.

- ACTIVE INGREDIENT
- PURPOSE
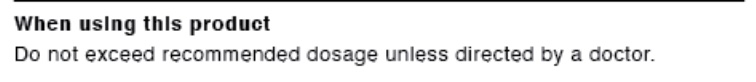
- WHEN USING
-
INSTRUCTIONS FOR USE
Directions
Adults: When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product. For products for external use only. Apply up to 6 times a day. Children under 12 years of age: consult a doctor.

-
INACTIVE INGREDIENT
Inactive Ingredients
Aacrylates, Allantoin, Arnice Montana Flower Extract, Deionized Water, Dimethyl Isosorbide, Emu Oil, Ethylhexyl Palmitate, Helianthus Annuus (Sunflower) Seed Oil, Hydrogenated Polydecene, Menthol, Phenoxythanol, Propylene Glycol, Sodium Polyacrylate, Tetrasodium Edta, Tocopheryl Acetate, Trideceth-6

- OTHER SAFETY INFORMATION
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SMART NUMB LIDOCAINE CREAM
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76092-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) MENTHOL (UNII: L7T10EIP3A) TRIDECETH-6 (UNII: 3T5PCR2H0C) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) SUNFLOWER OIL (UNII: 3W1JG795YI) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) HYDROGENATED POLYDECENE (1500 CST) (UNII: 4YI0729529) WATER (UNII: 059QF0KO0R) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) EMU OIL (UNII: 344821WD61) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) ETHYLHEXYL PALMITATE (UNII: 2865993309) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76092-201-02 57 g in 1 JAR; Type 0: Not a Combination Product 03/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/15/2024 Labeler - M.J. Winston International, Ltd. (144927378) Establishment Name Address ID/FEI Business Operations M.J. Winston International, Ltd. 144927378 label(76092-201)