Label: POSACONAZOLE tablet, delayed release
- NDC Code(s): 55154-4322-0
- Packager: Cardinal Health 107, LLC
- This is a repackaged label.
- Source NDC Code(s): 0904-7149
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POSACONAZOLE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for POSACONAZOLE DELAYED-RELEASE TABLETS.
POSACONAZOLE delayed-release tablets, for oral use.
Initial U.S. Approval: 2006RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Posaconazole is an azole antifungal indicated as follows: (1)
- •
- Posaconazole is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD)or those with hematologic malignancies with prolonged neutropenia from chemotherapy as follows: (1.1)
- •
- Posaconazole delayed-release tablets: adults and pediatric patients 13 years of age and older
DOSAGE AND ADMINISTRATION
- •
- Posaconazole oral suspension is not substitutable with posaconazole delayed-release tablets or posaconazole powdermix for delayed-release oral suspension due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations. (2.1, 2.2, 2.3, 2.8)
- •
- Administer Posaconazole delayed-release tablets with food. (2.1)
Table 1. Recommended Dosage in Adult Patients and Pediatric Patients aged 13 years and older Indication
Dose and Duration of Therapy
Prophylaxis of invasive Aspergillus and Candida infections
Delayed-Release Tablets:
Loading dose : 300 mg (three 100 mg delayed-release tablets) twice a day on the first day.
Maintenance dose : 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.2, 2.3)
DOSAGE FORMS AND STRENGTHS
- •
- Posaconazole delayed-release tablet 100 mg (3)
CONTRAINDICATIONS
- •
- Known hypersensitivity to posaconazole or other azole antifungal agents. (4.1)
- •
- Coadministration of posaconazole with the following drugs is contraindicated; posaconazole increases concentrations of:
- •
- Sirolimus: can result in sirolimus toxicity (4.2, 7.1)
- •
- CYP3A4 substrates (pimozide, quinidine): can result in QTc interval prolongation and cases of TdP (4.3, 7.2)
- •
- HMG-CoA Reductase Inhibitors Primarily Metabolized through CYP3A4: can lead to rhabdomyolysis (4.4, 7.3)
- •
- Ergot alkaloids: can result in ergotism (4.5, 7.4)
WARNINGS AND PRECAUTIONS
- •
- Calcineurin-Inhibitor Toxicity: posaconazole increases concentrations of cyclosporine or tacrolimus; reduce dose of cyclosporine and tacrolimus and monitor concentrations frequently. (5.1)
- •
- Arrhythmias and QTc Prolongation: posaconazole has been shown to prolong the QTc interval and cause cases of TdP. Administer with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs known to prolong QTc interval and metabolized through CYP3A4. (5.2)
- •
- Electrolyte Disturbances: Monitor and correct, especially those involving potassium (K+), magnesium (Mg++), and calcium (Ca++), before and during posaconazole therapy. (5.3)
- •
- Hepatic Toxicity: Elevations in liver tests may occur. Discontinuation should be considered in patients who develop abnormal liver tests or monitor liver tests during treatment. (5.4)
- •
- Concomitant use with Midazolam: posaconazole can prolong hypnotic/sedative effects. Monitor patients and benzodiazepine receptor antagonists should be available. (5.6, 7.5)
- •
- Vincristine Toxicity: Concomitant administration of azole antifungals, including posaconazole, with vincristine has been associated with neurotoxicity and other serious adverse reactions; reserve azole antifungals, including posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options. (5.7, 7.10)
- •
- Breakthrough Fungal Infections: Monitor patients with severe diarrhea or vomiting when receiving posaconazole delayed-release tablets. (5.9)
ADVERSE REACTIONS
- •
- Common adverse reactions in studies with posaconazole are diarrhea, nausea, fever, vomiting, headache, coughing, and hypokalemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AET Pharma US Inc, USA at 1 833-610-1604 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
Interaction Drug
Interaction
Rifabutin, phenytoin, efavirenz, cimetidine
Avoid coadministration unless the benefit outweighs the risks (7.6, 7.7, 7.8, 7.9)
Other drugs metabolized by CYP3A4
Consider dosage adjustment and monitor for adverse effects and toxicity (7.1, 7.10, 7.11)
Digoxin
Monitor digoxin plasma concentrations (7.12)
Fosamprenavir
USE IN SPECIFIC POPULATIONS
- •
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- •
- Pediatrics: Safety and effectiveness in patients younger than 2 years of age have not been established. (8.4)
- •
- Severe renal impairment: Monitor closely for breakthrough fungal infections. (8.6)
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) delayed-release tablets. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information. (8)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Invasive Aspergillus and Candida Infections
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing Regimen in Adult Patients
2.3 Dosing Regimen in Pediatric Patients (ages 13 to less than 18 years of age)
2.5 Administration Instructions for Posaconazole Delayed-Release Tablets
2.7 Non-Substitutability between Posaconazole Oral Suspension and Other Formulations
2.9 Dosage Adjustments in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Use with Sirolimus
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
4.5 Use with Ergot Alkaloids
5 WARNINGS AND PRECAUTIONS
5.1 Calcineurin-Inhibitor Drug Interactions
5.2 Arrhythmias and QT Prolongation
5.3 Electrolyte Disturbances
5.4 Hepatic Toxicity
5.5 Renal Impairment
5.6 Concomitant Use with Midazolam
5.7 Vincristine Toxicity
5.9 Breakthrough Fungal Infections
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Immunosuppressants Metabolized by CYP3A4
7.2 CYP3A4 Substrates
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
7.4 Ergot Alkaloids
7.5 Benzodiazepines Metabolized by CYP3A4
7.6 Anti-HIV Drugs
7.7 Rifabutin
7.8 Phenytoin
7.9 Gastric Acid Suppressors/Neutralizers
7.10 Vinca Alkaloids
7.11 Calcium Channel Blockers Metabolized by CYP3A4
7.12 Digoxin
7.13 Gastrointestinal Motility Agents
7.14 Glipizide
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Gender
8.9 Race
8.10 Weight
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Prophylaxis of Aspergillus and Candida Infections with Posaconazole Oral Suspension
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Invasive Aspergillus and Candida Infections
Posaconazole is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy [see Clinical Studies (14.1)] as follows:
Posaconazole delayed-release tablets: adults and pediatric patients 13 years of age and older.
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) delayed-release tablets. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Non-substitutable
[see Dosage and Administration (2.2,2.3).Posaconazole oral suspension is not substitutable with Posaconazole delayed-release tablets or Posaconazole powdermix for delayed-release oral suspension due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations
Posaconazole delayed-release tablets
- •
- Swallow tablets whole. Do not divide, crush, or chew.
- •
- Administer with food [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)] .
- •
- Posaconazole delayed-release tablets generally provide higher plasma drug exposures than posaconazole oral suspension under both fed and fasted conditions, and therefore is the preferred oral formulation for the prophylaxis indication
2.2 Dosing Regimen in Adult Patients
Table 1: Dosing Regimens in Adult Patients Indication
Dose and Frequency
Duration of Therapy
Prophylaxis of invasive Aspergillus and Candida infections
Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day.
Maintenance dose : 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day.
Loading dose:
1 day
Maintenance dose :
Duration of therapy is based on recovery from neutropenia or immunosuppression.
- •
- Swallow tablets whole. Do not divide, crush, or chew.
- •
- Administer posaconazole delayed-release tablets with food to enhance the oral absorption of posaconazole and optimize plasma concentrations [see Clinical Pharmacology (12.3)].
- •
- Posaconazole delayed-release tablets should be used only for the prophylaxis indication.
- •
- Posaconazole delayed-release tablets generally provide higher plasma drug exposures than posaconazole oral suspension under both fed and fasted conditions, and therefore is the preferred oral formulation for the prophylaxis indication.
2.3 Dosing Regimen in Pediatric Patients (ages 13 to less than 18 years of age)
The recommended dosing regimen of posaconazole for pediatric patients 13 to less than 18 years of age is shown in Table 2[see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
Table 2: Posaconazole delayed-release tablet Dosing Regimens for Pediatric Patients (Ages 2 to less than 18 years of age) Indication
Recomended Pediatric Dosage
Duration of Therapy
Prophylaxis of invasive Aspergillus and Candida infections
Loading dose: 300 mg twice daily on the first day.
Maintenance dose: 300 mg once daily.
Duration of therapy is based on recovery from neutropenia or immunosuppression.
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) delayed-release tablets. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.5 Administration Instructions for Posaconazole Delayed-Release Tablets
- •
- Swallow tablets whole. Do not divide, crush, or chew.
- •
- Administer posaconazole delayed-release tablets with food to enhance the oral absorption of posaconazole and optimize plasma concentrations [see Clinical Pharmacology (12.3)].
- •
- Posaconazole delayed-release tablets should be used only for the prophylaxis indication[see Dosage and Administration (2.1)].
2.7 Non-Substitutability between Posaconazole Oral Suspension and Other Formulations
Posaconazole oral suspension is not substitutable with posaconazole delayed-release tablets or posaconazole powdermix for delayed-release oral suspension due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations [see Dosage and Administration (2.2, 2.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Posaconazole is contraindicated in persons with known hypersensitivity to posaconazole or other azole antifungal agents.
4.2 Use with Sirolimus
Posaconazole is contraindicated with sirolimus. Concomitant administration of posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
Posaconazole is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of posaconazole with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
Coadministration with the HMG-CoA reductase inhibitors that are primarily metabolized through CYP3A4 (e.g., atorvastatin, lovastatin, and simvastatin) is contraindicated since increased plasma concentration of these drugs can lead to rhabdomyolysis [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
4.5 Use with Ergot Alkaloids
Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism [see Drug Interactions (7.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Calcineurin-Inhibitor Drug Interactions
Concomitant administration of posaconazole with cyclosporine or tacrolimus increases the whole blood trough concentrations of these calcineurin-inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Nephrotoxicity and leukoencephalopathy (including deaths) have been reported in clinical efficacy studies in patients with elevated cyclosporine or tacrolimus concentrations. Frequent monitoring of tacrolimus or cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus or cyclosporine dose adjusted accordingly.
5.2 Arrhythmias and QT Prolongation
Some azoles, including posaconazole, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, cases of torsades de pointes have been reported in patients taking posaconazole.
Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18-85 years of age) administered posaconazole oral suspension 400 mg twice daily with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc(F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc(F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered posaconazole had a QTc(F) interval ≥500 msec or an increase ≥60 msec in their QTc(F) interval from baseline.
Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)].
5.3 Electrolyte Disturbances
Electrolyte disturbances, especially those involving potassium, magnesium or calcium levels, should be monitored and corrected as necessary before and during posaconazole therapy.
5.4 Hepatic Toxicity
Hepatic reactions (e.g., mild to moderate elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and/or clinical hepatitis) have been reported in clinical trials. The elevations in liver tests were generally reversible on discontinuation of therapy, and in some instances these tests normalized without drug interruption. Cases of more severe hepatic reactions including cholestasis or hepatic failure including deaths have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with posaconazole. These severe hepatic reactions were seen primarily in subjects receiving the posaconazole oral suspension 800 mg daily (400 mg twice daily or 200 mg four times a day) in clinical trials.
Liver tests should be evaluated at the start of and during the course of posaconazole therapy. Patients who develop abnormal liver tests during posaconazole therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver tests and bilirubin). Discontinuation of posaconazole must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to posaconazole.
5.5 Renal Impairment
Due to the variability in exposure with Posaconazole delayed-release tablets, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2.9) and Use in Specific Populations (8.6)].
5.6 Concomitant Use with Midazolam
Concomitant administration of posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Patients must be monitored closely for adverse effects associated with high plasma concentrations of midazolam and benzodiazepine receptor antagonists must be available to reverse these effects [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
5.7 Vincristine Toxicity
Concomitant administration of azole antifungals, including posaconazole, with vincristine has been associated with neurotoxicity and other serious adverse reactions, including seizures, peripheral neuropathy, syndrome of inappropriate antidiuretic hormone secretion, and paralytic ileus. Reserve azole antifungals, including posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options [see Drug Interactions (7.10)].
-
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
- •
- Hypersensitivity [see Contraindications (4.1)]
- •
- Arrhythmias and QT Prolongation [see Warnings and Precautions (5.2)]
- •
- Hepatic Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of posaconazole cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Adults
Clinical Trial Experience with Posaconazole Delayed-Release Tablets
The safety of posaconazole delayed-release tablets has been assessed in 230 patients in clinical trials. Patients were enrolled in a non-comparative pharmacokinetic and safety trial of posaconazole delayed-release tablets when given as antifungal prophylaxis (Posaconazole Delayed-Release Tablet Study). Patients were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, GVHD, and post HSCT. This patient population was 62% male, had a mean age of 51 years (range 19-78 years, 17% of patients were ≥65 years of age), and were 93% white and 16% Hispanic. Posaconazole therapy was given for a median duration of 28 days. Twenty patients received 200 mg daily dose and 210 patients received 300 mg daily dose (following twice daily dosing on Day 1 in each cohort).
Table 8: presents adverse reactions observed in patients treated with 300 mg daily dose at an incidence of > 10% in posaconazole delayed-release tablet study.
Table 8: Posaconazole Delayed-Release Tablet Study: Adverse Reactions in at Least 10% of Subjects Treated with 300 mg Daily Dose Body System
Posaconazole delayed-release tablet (300 mg)
n=210 (%)
Subjects Reporting any Adverse Reaction
207
(99)
Blood and Lymphatic System Disorder
Anemia
22
(10)
Thrombocytopenia
29
(14)
Gastrointestinal Disorders
Abdominal Pain
23
(11)
Constipation
20
(10)
Diarrhea
61
(29)
Nausea
56
(27)
Vomiting
28
(13)
General Disorders and Administration Site Conditions
Asthenia
20
(10)
Chills
22
(10)
Mucosal Inflammation
29
(14)
Edema Peripheral
33
(16)
Pyrexia
59
(28)
Metabolism and Nutrition Disorders
Hypokalemia
46
(22)
Hypomagnesemia
20
(10)
Nervous System Disorders
Headache
30
(14)
Respiratory, Thoracic and Mediastinal Disorders
Cough
35
(17)
Epistaxis
30
(14)
Skin and Subcutaneous Tissue Disorders
Rash
34
(16)
Vascular Disorders
Hypertension
23
(11)
The most frequently reported adverse reactions (>25%) with posaconazole delayed-release tablets 300 mg once daily were diarrhea, pyrexia, and nausea.
The most common adverse reaction leading to discontinuation of posaconazole delayed-release tablets 300 mg once daily was nausea (2%).
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) products. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
6.2 Postmarketing Experience
The following adverse reaction has been identified during the post-approval use of posaconazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
Endocrine Disorders: Pseudoaldosteronism
-
7 DRUG INTERACTIONS
Posaconazole is primarily metabolized via UDP glucuronosyltransferase and is a substrate of p -glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Coadministration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole [see Clinical Pharmacology (12.3)].
The following information was derived from data with posaconazole oral suspension or early tablet formulation otherwise noted.
7.1 Immunosuppressants Metabolized by CYP3A4
Sirolimus: Concomitant administration of posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity. Therefore, posaconazole is contraindicated with sirolimus [see Contraindications (4.2) and Clinical Pharmacology (12.3)].
Tacrolimus: Posaconazole has been shown to significantly increase the Cmax and AUC of tacrolimus. At initiation of posaconazole treatment, reduce the tacrolimus dose to approximately one-third of the original dose. Frequent monitoring of tacrolimus whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
Cyclosporine: Posaconazole has been shown to increase cyclosporine whole blood concentrations in heart transplant patients upon initiation of posaconazole treatment. It is recommended to reduce cyclosporine dose to approximately three-fourths of the original dose upon initiation of posaconazole treatment. Frequent monitoring of cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the cyclosporine dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 CYP3A4 Substrates
Concomitant administration of posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, posaconazole is contraindicated with these drugs [see Contraindications (4.3) and Warnings and Precautions (5.2)].
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
Concomitant administration of posaconazole with simvastatin increases the simvastatin plasma concentrations by approximately 10-fold. Therefore, posaconazole is contraindicated with HMG-CoA reductase inhibitors primarily metabolized through CYP3A4 [see Contraindications (4.4) and Clinical Pharmacology (12.3)].
7.4 Ergot Alkaloids
Most of the ergot alkaloids are substrates of CYP3A4. Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism. Therefore, posaconazole is contraindicated with ergot alkaloids [see Contraindications (4.5)].
7.5 Benzodiazepines Metabolized by CYP3A4
Concomitant administration of posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant use of posaconazole and other benzodiazepines metabolized by CYP3A4 (e.g., alprazolam, triazolam) could result in increased plasma concentrations of these benzodiazepines. Patients must be monitored closely for adverse effects associated with high plasma concentrations of benzodiazepines metabolized by CYP3A4 and benzodiazepine receptor antagonists must be available to reverse these effects [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.6 Anti-HIV Drugs
Efavirenz: Efavirenz induces UDP-glucuronidase and significantly decreases posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. It is recommended to avoid concomitant use of efavirenz with posaconazole unless the benefit outweighs the risks.
Ritonavir and Atazanavir: Ritonavir and atazanavir are metabolized by CYP3A4 and posaconazole increases plasma concentrations of these drugs [see Clinical Pharmacology (12.3)]. Frequent monitoring of adverse effects and toxicity of ritonavir and atazanavir should be performed during coadministration with posaconazole.
Fosamprenavir: Combining fosamprenavir with posaconazole may lead to decreased posaconazole plasma concentrations. If concomitant administration is required, close monitoring for breakthrough fungal infections is recommended [see Clinical Pharmacology (12.3)].
7.7 Rifabutin
Rifabutin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Rifabutin is also metabolized by CYP3A4. Therefore, coadministration of rifabutin with posaconazole increases rifabutin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of posaconazole and rifabutin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections as well as frequent monitoring of full blood counts and adverse reactions due to increased rifabutin plasma concentrations (e.g., uveitis, leukopenia) are recommended.
7.8 Phenytoin
Phenytoin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Phenytoin is also metabolized by CYP3A4. Therefore, coadministration of phenytoin with posaconazole increases phenytoin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of posaconazole and phenytoin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections is recommended and frequent monitoring of phenytoin concentrations should be performed while coadministered with posaconazole and dose reduction of phenytoin should be considered.
7.9 Gastric Acid Suppressors/Neutralizers
No clinically relevant effects on the pharmacokinetics of posaconazole were observed when posaconazole delayed-release tablets are concomitantly used with antacids, H2-receptor antagonists and proton pump inhibitors [see Clinical Pharmacology (12.3)]. No dosage adjustment of posaconazole delayed-release tablets is required when posaconazole delayed-release tablets are concomitantly used with antacids, H2-receptor antagonists and proton pump inhibitors.
7.10 Vinca Alkaloids
Most of the vinca alkaloids (e.g., vincristine and vinblastine) are substrates of CYP3A4. Concomitant administration of azole antifungals, including posaconazole, with vincristine has been associated with serious adverse reactions [see Warnings and Precautions (5.7)]. Posaconazole may increase the plasma concentrations of vinca alkaloids which may lead to neurotoxicity and other serious adverse reactions. Therefore, reserve azole antifungals, including posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options.
7.11 Calcium Channel Blockers Metabolized by CYP3A4
Posaconazole may increase the plasma concentrations of calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Frequent monitoring for adverse reactions and toxicity related to calcium channel blockers is recommended during coadministration. Dose reduction of calcium channel blockers may be needed.
7.12 Digoxin
Increased plasma concentrations of digoxin have been reported in patients receiving digoxin and posaconazole. Therefore, monitoring of digoxin plasma concentrations is recommended during coadministration.
7.13 Gastrointestinal Motility Agents
Concomitant administration of metoclopramide with posaconazole delayed-release tablets did not affect the pharmacokinetics of posaconazole [see Clinical Pharmacology (12.3)]. No dosage adjustment of posaconazole delayed-release tablets is required when given concomitantly with metoclopramide.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal data, posaconazole may cause fetal harm when administered to pregnant women. Available data for use of posaconazole in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, skeletal malformations (cranial malformations and missing ribs) and maternal toxicity (reduced food consumption and reduced body weight gain) were observed when posaconazole was dosed orally to pregnant rats during organogenesis at doses ≥1.4 times the 400 mg twice daily oral suspension regimen based on steady-state plasma concentrations of posaconazole in healthy volunteers. In pregnant rabbits dosed orally during organogenesis, increased resorptions, reduced litter size, and reduced body weight gain of females were seen at doses 5 times the exposure achieved with the 400 mg twice daily oral suspension regimen. Doses of ≥ 3 times the clinical exposure caused an increase in resorptions in these rabbits (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Posaconazole resulted in maternal toxicity (reduced food consumption and reduced body weight gain) and skeletal malformations (cranial malformations and missing ribs) when given orally to pregnant rats during organogenesis (Gestational Days 6 through 15) at doses ≥27 mg/kg (≥1.4 times the 400 mg twice daily oral suspension regimen based on steady-state plasma concentrations of drug in healthy volunteers). The no-effect dose for malformations and maternal toxicity in rats was 9 mg/kg, which is 0.7 times the exposure achieved with the 400 mg twice daily oral suspension regimen. No malformations were seen in rabbits dosed during organogenesis (Gestational Days 7 through 19) at doses up to 80 mg/kg (5 times the exposure achieved with the 400 mg twice daily oral suspension regimen). In the rabbit, the no-effect dose was 20 mg/kg, while high doses of 40 mg/kg and 80 mg/kg (3 or 5 times the clinical exposure) caused an increase in resorptions. In rabbits dosed at 80 mg/kg, a reduction in body weight gain of females and a reduction in litter size were seen.
8.2 Lactation
Risk Summary
There are no data on the presence of posaconazole in human milk, the effects on the breastfed infant, or the effects on milk production. Posaconazole is excreted in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for posaconazole and any potential adverse effects on the breastfed child from posaconazole or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of posaconazole delayed-release tablets for the prophylaxis of invasive Aspergillus and Candida infections have been established in pediatric patients aged 13 years and older who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with GVHD or those with hematologic malignancies with prolonged neutropenia from chemotherapy.
Use of posaconazole in these age groups is supported by evidence from adequate and well-controlled studies of posaconazole in adult and pediatric patients and additional pharmacokinetic and safety data in pediatric patients 13 years of age and older [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14)].
The safety and effectiveness of posaconazole have not been established in pediatric patients younger than 2 years of age.
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) delayed-release tablets. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
No overall differences in the safety of posaconazole delayed-release tablets were observed between geriatric patients and younger adult patients in the clinical trials; therefore, no dosage adjustment is recommended for any formulation of posaconazole in geriatric patients. No clinically meaningful differences in the pharmacokinetics of posaconazole were observed in geriatric patients compared to younger adult patients during clinical trials [see Clinical Pharmacology (12.3)].
Of the 230 patients treated with posaconazole delayed-release tablets, 38 (17%) were greater than 65 years of age.
No overall differences in the pharmacokinetics and safety were observed between elderly and young subjects during clinical trials, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Following single-dose administration of 400 mg of the posaconazole oral suspension, there was no significant effect of mild (eGFR: 50-80 mL/min/1.73 m2, n=6) or moderate (eGFR: 20-49 mL/min/1.73 m2, n=6) renal impairment on posaconazole pharmacokinetics; therefore, no dose adjustment is required in patients with mild to moderate renal impairment. In subjects with severe renal impairment (eGFR: <20 mL/min/1.73 m2), the mean plasma exposure (AUC) was similar to that in patients with normal renal function (eGFR: >80 mL/min/1.73 m2); however, the range of the AUC estimates was highly variable (CV=96%) in these subjects with severe renal impairment as compared to that in the other renal impairment groups (CV<40%). Due to the variability in exposure, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2.9)]. Similar recommendations apply to posaconazole delayed-release tablets; however, a specific study has not been conducted with the posaconazole delayed-release tablets.
8.7 Hepatic Impairment
After a single oral dose of posaconazole oral suspension 400 mg, the mean AUC was 43%, 27%, and 21% higher in subjects with mild (Child-Pugh Class A, N=6), moderate (Child-Pugh Class B, N=6), or severe (Child-Pugh Class C, N=6) hepatic impairment, respectively, compared to subjects with normal hepatic function (N=18). Compared to subjects with normal hepatic function, the mean Cmax was 1% higher, 40% higher, and 34% lower in subjects with mild, moderate, or severe hepatic impairment, respectively. The mean apparent oral clearance (CL/F) was reduced by 18%, 36%, and 28% in subjects with mild, moderate, or severe hepatic impairment, respectively, compared to subjects with normal hepatic function. The elimination half-life (t½) was 27 hours, 39 hours, 27 hours, and 43 hours in subjects with normal hepatic function and mild, moderate, or severe hepatic impairment, respectively.
It is recommended that no dose adjustment of posaconazole is needed in patients with mild to severe hepatic impairment (Child-Pugh Class A, B, or C) [see Dosage and Administration (2) and Warnings and Precautions (5.4)]. Similar recommendations apply to posaconazole delayed-release tablets; however, a specific study has not been conducted with the delayed-release tablets.
8.8 Gender
The pharmacokinetics of posaconazole are comparable in males and females. No adjustment in the dosage of posaconazole is necessary based on gender.
-
10 OVERDOSAGE
There is no experience with overdosage of delayed-release tablets.
During the clinical trials, some patients received posaconazole oral suspension up to 1600 mg/day with no adverse reactions noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg twice daily posaconazole oral suspension for 3 days. No related adverse reactions were noted by the investigator.
Posaconazole is not removed by hemodialysis.
-
11 DESCRIPTION
Posaconazole is an azole antifungal agent. Posaconazole is available as delayed-release tablet intended for oral administration.
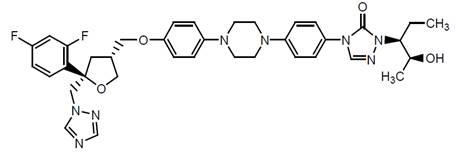
Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (2,4-difluorophenyl)tetrahydro-5 (1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2 hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one with an empirical formula of C37H42F2N8O4 and a molecular weight of 700.8. The chemical structure is:
Posaconazole is a white powder with a low aqueous solubility.
Posaconazole delayed-release tablets are yellow, coated, capsule-shaped tablets containing 100 mg of posaconazole. Each delayed-release tablet contains the inactive ingredients: partially neutralized methacrylic acid and ethyl acrylate copolymer, triethyl citrate, xylitol, hydroxypropyl cellulose, propyl gallate, cellulose, microcrystalline, silica, colloidal anhydrous, croscarmellose sodium, sodium stearyl fumarate and Opadry® II Yellow (consists of the following ingredients: polyvinyl alcohol partially hydrolyzed, macrogol, polyethylene glycol, titanium dioxide, talc, and iron oxide yellow).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Posaconazole is an azole antifungal agent [see Clinical Pharmacology (12.4)].
12.2 Pharmacodynamics
Exposure Response Relationship: In clinical studies of neutropenic patients who were receiving cytotoxic chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) or hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD), a wide range of plasma exposures to posaconazole was noted following administration of posaconazole oral suspension. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cavg) and prophylactic efficacy (Table 15). A lower Cavg may be associated with an increased risk of treatment failure, defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections.
Table 15: Posaconazole Oral Suspension Exposure Analysis (Cavg) in Prophylaxis Trials Cavg = the average posaconazole concentration when measured at steady state Prophylaxis in AML/MDS*
Prophylaxis in GVHD†
Cavg Range (ng/mL)
Treatment Failure‡ (%)
Cavg Range (ng/mL)
Treatment Failure‡ (%)
Quartile 1
90-322
54.7
22-557
44.4
Quartile 2
322-490
37.0
557-915
20.6
Quartile 3
490-734
46.8
915-1563
17.5
Quartile 4
734-2200
27.8
1563-3650
17.5
Cavg = the average posaconazole concentration when measured at steady state
* Neutropenic patients who were receiving cytotoxic chemotherapy for AML or MDS
† HSCT recipients with GVHD
‡ Defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections
12.3 Pharmacokinetics
General Pharmacokinetic Characteristics
Posaconazole delayed-release tablets exhibit dose proportional pharmacokinetics after single and multiple dosing up to 300 mg. The mean pharmacokinetic parameters of posaconazole at steady state following administration of posaconazole delayed-release tablets 300 mg twice daily on Day 1, then 300 mg once daily thereafter in healthy volunteers and in neutropenic patients who are receiving cytotoxic chemotherapy for AML or MDS or HSCT recipients with GVHD are shown in Table 18.
Table 18: Arithmetic Mean (%CV) of Steady State PK Parameters in Healthy Volunteers and Patients Following Administration of Posaconazole Delayed-Release Tablets (300 mg)* CV = coefficient of variation expressed as a percentage (%CV); AUC0-T = Area under the plasma concentration-time curve from time zero to 24 hr; Cmax = maximum observed concentration; Cmin = minimum observed plasma concentration; Tmax = time of maximum observed concentration; t½ = terminal phase half-life; CL /F = Apparent total body clearance N
AUC 0-24 hr
(ng·hr/mL)
Cav† (ng/mL)
C max (ng/mL)
C min (ng/mL)
‡Tmax(hr)
t 1/2 (hr)
CL/F
(L/hr)
Healthy Volunteers
12
51618
(25)
2151
(25)
2764
(21)
1785
(29)
4
(3-6)
31
(40)
7.5
(26)
Patients
50
37900
(42)
1580
(42)
2090
(38)
1310
(50)
4 (1.3-8.3)
-
9.39
(45)
CV = coefficient of variation expressed as a percentage (%CV); AUC0-T = Area under the plasma concentration-time curve from time zero to 24 hr; Cmax = maximum observed concentration; Cmin = minimum observed plasma concentration; Tmax = time of maximum observed concentration; t½ = terminal phase half-life; CL /F = Apparent total body clearance
*300 mg twice daily on Day 1, then 300 mg once daily thereafter
† Cav = time-averaged concentrations (i.e., AUC0-24 hr/24hr)
‡ Median (minimum-maximum)
Absorption:
When given orally in healthy volunteers, posaconazole delayed-release tablets are absorbed with a median Tmax of 4 to 5 hours. Steady-state plasma concentrations are attained by Day 6 at the 300 mg dose (once daily after twice daily loading dose at Day 1). The absolute bioavailability of the oral delayed-release tablet is approximately 54% under fasted conditions. The Cmax and AUC of posaconazole following administration of posaconazole delayed-release tablets is increased 16% and 51%, respectively, when given with a high fat meal compared to a fasted state (see Table 20). In order to enhance the oral absorption of posaconazole and optimize plasma concentrations, posaconazole delayed-release tablets should be administered with food.
Table 20: Statistical Comparison of Plasma Pharmacokinetics of Posaconazole Following Single Oral Dose Administration of 300 mg Posaconazole Delayed-Release Tablet to Healthy Subjects under Fasting and Fed Conditions GMR=Geometric least-squares mean ratio; CI=Confidence interval Fasting Conditions
Fed Conditions (High Fat Meal)*
Fed/Fasting
Pharmacokinetic Parameter
N
Mean (%CV)
N
Mean (%CV)
GMR (90% CI)
Cmax (ng/mL)
14
935 (34)
16
1060 (25)
1.16 (0.96, 1.41)
AUC0-72hr (hr∙ng/mL)
14
26200 (28)
16
38400 (18)
1.51 (1.33, 1.72)
Tmax† (hr)
14
5.00 (3.00, 8.00)
16
6.00 (5.00, 24.00)
N/A
GMR=Geometric least-squares mean ratio; CI=Confidence interval
* 48.5 g fat
† Median (Min, Max) reported for Tmax
Concomitant administration of posaconazole delayed-release tablets with drugs affecting gastric pH or gastric motility did not demonstrate any significant effects on posaconazole pharmacokinetic exposure (see Table 21).
Table 21: The Effect of Concomitant Medications that Affect the Gastric pH and Gastric Motility on the Pharmacokinetics of Posaconazole Delayed-Release Tablets in Healthy Volunteers Coadministered Drug
Administration Arms
Change in Cmax (ratio estimate*; 90% CI of the ratio estimate)
Change in AUC0-last (ratio estimate*; 90% CI of the ratio estimate)
Mylanta® Ultimate strength liquid (Increase in gastric pH)
25.4 meq/5 mL, 20 mL
↑6%
(1.06; 0.90 -1.26)↑
↑4%
(1.04; 0.90 -1.20)
Ranitidine (Zantac®) (Alteration in gastric pH)
150 mg (morning dose of 150 mg Ranitidine twice daily)
↑4%
(1.04; 0.88 -1.23)↑
↓3%
(0.97; 0.84 -1.12)
Esomeprazole (Nexium®) (Increase in gastric pH)
40 mg (every morning for 5 days, Day -4 to 1)
↑2%
(1.02; 0.88-1.17)↑
↑5%
(1.05; 0.89 -1.24)
Metoclopramide (Reglan®) (Increase in gastric motility)
15 mg four times daily for 2 days (Day -1 and 1)
↓14% (0.86, 0.73,1.02)
↓7%
(0.93, 0.803,1.07)
* Ratio Estimate is the ratio of coadministered drug plus posaconazole to posaconazole alone for Cmax or AUC0-last.
Distribution:
Posaconazole is highly bound to human plasma proteins (>98%), predominantly to albumin.
Metabolism:
Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Posaconazole is primarily metabolized via UDP glucuronidation (phase 2 enzymes) and is a substrate for p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. A summary of drugs studied clinically with the oral suspension or an early tablet formulation, which affect posaconazole concentrations, is provided in Table 25.
Table 25: Summary of the Effect of Coadministered Drugs on Posaconazole in Healthy Volunteers Coadministered Drug (Postulated
Mechanism of Interaction)
Coadministered Drug Dose/Schedule
Posaconazole Dose/Schedule
Effect on Bioavailability of Posaconazole
Change in Mean Cmax
(ratio estimate*; 90%
CI of the ratio estimate)
Change in Mean AUC
(ratio estimate*; 90%
CI of the ratio estimate)
Efavirenz
(UDP-G Induction)
400 mg once daily × 10 and 20 days
400 mg (oral suspension) twice daily × 10 and 20 days
↓45%
(0.55; 0.47-0.66)
↓45%
(0.55; 0.47-0.66)
Fosamprenavir (unknown mechanism)
700 mg twice daily x 10 days
200 mg once daily on the 1st day, 200 mg twice daily on the 2nd day, then 400 mg twice daily x 8 Days
↓21%
0.79 (0.71-0.89)
↓23%
0.77 (0.68-0.87)
Rifabutin
(UDP-G Induction)
300 mg once daily x 17 days
200 mg (tablets) once daily
× 10 days†
↓ 43%
(0.57; 0.43-0.75)
↓ 49%
(0.51; 0.37-0.71)
Phenytoin
(UDP-G Induction)
200 mg once daily x 10 days
200 mg (tablets) once daily
× 10 days†
↓ 41%
(0.59; 0.44-0.79)
↓ 50%
(0.50; 0.36-0.71)
* Ratio Estimate is the ratio of coadministered drug plus posaconazole to posaconazole alone for Cmax or AUC.
† The tablet refers to a non-commercial tablet formulation without polymer.
In vitro studies with human hepatic microsomes and clinical studies indicate that posaconazole is an inhibitor primarily of CYP3A4. A clinical study in healthy volunteers also indicates that posaconazole is a strong CYP3A4 inhibitor as evidenced by a >5-fold increase in midazolam AUC. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole. A summary of the drugs studied clinically, for which plasma concentrations were affected by posaconazole, is provided in Table 26 [see Contraindications (4) and Drug Interactions (7.1) including recommendations].
Table 26: Summary of the Effect of Posaconazole on Coadministered Drugs in Healthy Volunteers and Patients Coadministered Drug (Postulated Mechanism of Interaction is Inhibition of CYP3A4 by posaconazole)
Coadministered Drug Dose/Schedule
Posaconazole Dose/ Schedule
Effect on Bioavailability of Coadministered
Drugs
Change in Mean Cmax
(ratio estimate*;
90% CI of the ratio estimate)
Change in Mean AUC
(ratio estimate*; 90% CI of the ratio estimate)
Sirolimus
2-mg single oral dose
400 mg (oral suspension)
twice daily x 16 days
↑ 572%
(6.72; 5.62-8.03)
↑ 788%
(8.88; 7.26-10.9)
Cyclosporine
Stable maintenance dose in heart transplant recipients
200 mg (tablets)
once daily x 10 days†
↑ cyclosporine whole blood trough concentrations
Cyclosporine dose reductions of up to 29% were required
Tacrolimus
0.05-mg/kg single oral dose
400 mg (oral suspension)
twice daily × 7 days
↑ 121%
(2.21; 2.01-2.42)
↑ 358%
(4.58; 4.03-5.19)
Simvastatin
40-mg single oral dose
100 mg (oral suspension)
once daily x 13 days
Simvastatin
↑ 841%
(9.41, 7.13-12.44)
Simvastatin Acid
↑ 817%
(9.17, 7.36-11.43)
Simvastatin
↑ 931%
(10.31, 8.40-12.67)
Simvastatin Acid
↑ 634%
(7.34, 5.82-9.25)
200 mg (oral suspension)
once daily x 13 days
Simvastatin
↑ 1041%
(11.41, 7.99-16.29)
Simvastatin Acid
↑ 851%
(9.51, 8.15-11.10)
Simvastatin
↑ 960%
(10.60, 8.63-13.02)
Simvastatin Acid
↑748%
(8.48, 7.04-10.23)
Midazolam
0.4-mg single
intravenous dose‡
200 mg (oral suspension)
twice daily x 7 days
↑ 30%
(1.3; 1.13-1.48)
↑ 362%
(4.62; 4.02-5.3)
0.4-mg single
intravenous dose‡
400 mg (oral suspension)
twice daily x 7 days
↑62
(1.62; 1.41-1.86)
↑524%
(6.24; 5.43-7.16)
2-mg single oral dose‡
200 mg (oral suspension) once daily x 7 days
↑ 169%
(2.69; 2.46-2.93)
↑ 470%
(5.70; 4.82-6.74)
2-mg single oral dose‡
400 mg (oral suspension) twice daily x 7 days
↑ 138%
(2.38; 2.13-2.66)
↑ 397%
(4.97; 4.46-5.54)
Rifabutin
300 mg once daily x 17 days
200 mg (tablets)
once daily × 10 days†
↑ 31%
(1.31; 1.10-1.57)
↑ 72%
(1.72;1.51-1.95)
Phenytoin
200 mg once daily PO x 10 days
200 mg (tablets)
once daily x 10 days†
↑ 16%
(1.16; 0.85-1.57)
↑ 16%
(1.16; 0.84-1.59)
Ritonavir
100 mg once daily x 14 days
400 mg (oral suspension) twice daily x 7 days
↑ 49%
(1.49; 1.04-2.15)
↑ 80%
(1.8;1.39-2.31)
Atazanavir
Atazanavir/
ritonavir boosted
regimen
300 mg once daily x 14 days
400 mg (oral suspension)
twice daily x 7 days
↑ 155%
(2.55; 1.89-3.45)
↑ 268%
(3.68; 2.89-4.70)
300 mg/100 mg once daily x 14 days
400 mg (oral suspension)
twice daily x 7 days
↑ 53%
(1.53; 1.13-2.07)
↑ 146%
(2.46; 1.93-3.13)
* Ratio Estimate is the ratio of coadministered drug plus posaconazole to coadministered drug alone for Cmax or AUC.
† The tablet refers to a non-commercial tablet formulation without polymer.
‡ The mean terminal half-life of midazolam was increased from 3 hours to 7 to 11 hours during coadministration with posaconazole.
Additional clinical studies demonstrated that no clinically significant effects on zidovudine, lamivudine, indinavir, or caffeine were observed when administered with posaconazole 200 mg once daily; therefore, no dose adjustments are required for these coadministered drugs when coadministered with posaconazole 200 mg once daily.
Excretion:
Posaconazole delayed-release tablets are eliminated with a mean half-life (t½) ranging between 26 to 31 hours.
Pediatric Patients
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) delayed-release tablets. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
12.4 Microbiology
Mechanism of Action:
Posaconazole blocks the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14α-demethylase responsible for the conversion of lanosterol to ergosterol in the fungal cell membrane. This results in an accumulation of methylated sterol precursors and a depletion of ergosterol within the cell membrane thus weakening the structure and function of the fungal cell membrane. This may be responsible for the antifungal activity of posaconazole.
Resistance:
Clinical isolates of Candida albicans and Candida glabrata with decreased susceptibility to posaconazole were observed in oral swish samples taken during prophylaxis with posaconazole and fluconazole, suggesting a potential for development of resistance. These isolates also showed reduced susceptibility to other azoles, suggesting cross-resistance between azoles. The clinical significance of this finding is not known.
Antimicrobial Activity:
Posaconazole has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Microorganisms:
Aspergillus spp. and Candida spp.
Susceptibility Testing:
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see:
https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No drug-related neoplasms were recorded in rats or mice treated with posaconazole for 2 years at doses higher than the clinical dose. In a 2-year carcinogenicity study, rats were given posaconazole orally at doses up to 20 mg/kg (females), or 30 mg/kg (males). These doses are equivalent to 3.9- or 3.5-times the exposure achieved with a 400-mg twice daily oral suspension regimen, respectively, based on steady-state AUC in healthy volunteers administered a high-fat meal (400-mg twice daily oral suspension regimen). In the mouse study, mice were treated at oral doses up to 60 mg/kg/day or 4.8-times the exposure achieved with a 400-mg twice daily oral suspension regimen.
Mutagenesis
Posaconazole was not genotoxic or clastogenic when evaluated in bacterial mutagenicity (Ames), a chromosome aberration study in human peripheral blood lymphocytes, a Chinese hamster ovary cell mutagenicity study, and a mouse bone marrow micronucleus study.
Impairment of Fertility
Posaconazole had no effect on fertility of male rats at a dose up to 180 mg/kg (1.7 x the 400-mg twice daily oral suspension regimen based on steady-state plasma concentrations in healthy volunteers) or female rats at a dose up to 45 mg/kg (2.2 x the 400-mg twice daily oral suspension regimen).
13.2 Animal Toxicology and/or Pharmacology
In a nonclinical study using intravenous administration of posaconazole in very young dogs (dosed from 2 to 8 weeks of age), an increase in the incidence of brain ventricle enlargement was observed in treated animals as compared with concurrent control animals. No difference in the incidence of brain ventricle enlargement between control and treated animals was observed following the subsequent 5 month treatment-free period. There were no neurologic, behavioral or developmental abnormalities in the dogs with this finding, and a similar brain finding was not seen with oral posaconazole administration to juvenile dogs (4 days to 9 months of age). There were no drug-related increases in the incidence of brain ventricle enlargement when treated and control animals were compared in a separate study of 10-week old dogs dosed with intravenous posaconazole for 13 weeks with a 9-week recovery period or a follow-up study of 31-week old dogs dosed for 3 months.
-
14 CLINICAL STUDIES
14.1 Prophylaxis of Aspergillus and Candida Infections with Posaconazole Oral Suspension
Two randomized, controlled studies were conducted using posaconazole as prophylaxis for the prevention of invasive fungal infections (IFIs) among patients at high risk due to severely compromised immune systems.
The first study (Oral Suspension Study 1) was a randomized, double-blind trial that compared posaconazole oral suspension (200 mg three times a day) with fluconazole capsules (400 mg once daily) as prophylaxis against invasive fungal infections in allogeneic hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD). Efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (patients may have met more than one of these criteria). This assessed all patients while on study therapy plus 7 days and at 16 weeks post-randomization. The mean duration of therapy was comparable between the 2 treatment groups (80 days, posaconazole; 77 days, fluconazole). Table 28 contains the results from Oral Suspension Study 1.
Table 28: Results from Blinded Clinical Study in Prophylaxis of IFI in All Randomized Patients with Hematopoietic Stem Cell Transplant (HSCT) and Graft-vs.-Host Disease (GVHD): Oral Suspension Study 1 Posaconazole n=301
Fluconazole n=299
On therapy plus 7 days
Clinical Failure*
50 (17%)
55 (18%)
Failure due to:
Proven/Probable IFI
7 (2%)
22 (7%)
(Aspergillus )
3 (1%)
17 (6%)
(Candida )
1 (<1%)
3 (1%)
(Other)
3 (1%)
2 (1%)
All Deaths
Proven/probable fungal
infection prior to death
22 (7%)
2 (<1%)
24 (8%)
6 (2%)
SAF†
27 (9%)
25 (8%)
Through 16 weeks
Clinical Failure*,‡
99 (33%)
110 (37%)
Failure due to:
Proven/Probable IFI
16 (5%)
27 (9%)
(Aspergillus )
7 (2%)
21 (7%)
(Candida )
4 (1%)
4 (1%)
(Other)
5 (2%)
2 (1%)
All Deaths
Proven/probable fungal
infection prior to death
58 (19%)
10 (3%)
59 (20%)
16 (5%)
SAF†
26 (9%)
30 (10%)
Event free lost to follow-up§
24 (8%)
30 (10%)
* Patients may have met more than one criterion defining failure.
† Use of systemic antifungal therapy (SAF) criterion is based on protocol definitions (empiric/IFI usage >4 consecutive days).
‡ 95% confidence interval (posaconazole-fluconazole) = (-11.5%, +3.7%).
§ Patients who are lost to follow-up (not observed for 112 days), and who did not meet another clinical failure endpoint. These patients were considered failures.
The second study (Oral Suspension Study 2) was a randomized, open-label study that compared posaconazole oral suspension (200 mg 3 times a day) with fluconazole suspension (400 mg once daily) or itraconazole oral solution (200 mg twice a day) as prophylaxis against IFIs in neutropenic patients who were receiving cytotoxic chemotherapy for AML or MDS. As in Oral Suspension Study 1, efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (Patients might have met more than one of these criteria). This study assessed patients while on treatment plus 7 days and 100 days postrandomization. The mean duration of therapy was comparable between the 2 treatment groups (29 days, posaconazole; 25 days, fluconazole or itraconazole). Table 29 contains the results from Oral Suspension Study 2.
Table 29: Results from Open-Label Clinical Study 2 in Prophylaxis of IFI in All Randomized Patients with Hematologic Malignancy and Prolonged Neutropenia: Oral Suspension Study 2 Posaconazole n=304
Fluconazole/Itraconazole n=298
On therapy plus 7 days
Clinical Failure*,†
82 (27%)
126 (42%)
Failure due to:
Proven/Probable IFI
7 (2%)
25 (8%)
(Aspergillus )
2 (1%)
20 (7%)
(Candida )
3 (1%)
2 (1%)
(Other)
2 (1%)
3 (1%)
All Deaths
Proven/probable fungal
infection prior to death
17 (6%)
1 (<1%)
25 (8%)
2 (1%)
SAF‡
67 (22%)
98 (33%)
Through 100 days postrandomization
Clinical Failure†
158 (52%)
191 (64%)
Failure due to:
Proven/Probable IFI
14 (5%)
33 (11%)
(Aspergillus )
2 (1%)
26 (9%)
(Candida )
10 (3%)
4 (1%)
(Other)
2 (1%)
3 (1%)
All Deaths
Proven/probable fungal
infection prior to death
44 (14%)
2 (1%)
64 (21%)
16 (5%)
SAF‡
98 (32%)
125 (42%)
Event free lost to follow-up§
34 (11%)
24 (8%)
* 95% confidence interval (posaconazole-fluconazole/itraconazole) = (-22.9%, -7.8%).
† Patients may have met more than one criterion defining failure.
‡ Use of systemic antifungal therapy (SAF) criterion is based on protocol definitions (empiric/IFI usage >3 consecutive days).
§ Patients who are lost to follow-up (not observed for 100 days), and who did not meet another clinical failure endpoint. These patients were considered failures.
In summary, 2 clinical studies of prophylaxis were conducted with the posaconazole oral suspension. As seen in the accompanying tables (Tables 28 and 29), clinical failure represented a composite endpoint of breakthrough IFI, mortality and use of systemic antifungal therapy. In Oral Suspension Study 1 (Table 28), the clinical failure rate of posaconazole (33%) was similar to fluconazole (37%), (95% CI for the difference posaconazole–comparator -11.5% to 3.7%) while in Oral Suspension Study 2 (Table 29) clinical failure was lower for patients treated with posaconazole (27%) when compared to patients treated with fluconazole or itraconazole (42%), (95% CI for the difference posaconazole–comparator -22.9% to 7.8%).
All-cause mortality was similar at 16 weeks for both treatment arms in Oral Suspension Study 1 [POS 58/301 (19%) vs. FLU 59/299 (20%)]; all-cause mortality was lower at 100 days for posaconazole-treated patients in Oral Suspension Study 2 [POS 44/304 (14%) vs. FLU/ITZ 64/298 (21%)]. Both studies demonstrated fewer breakthrough infections caused by Aspergillus species in patients receiving posaconazole prophylaxis when compared to patients receiving fluconazole or itraconazole.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Important Administration Instructions
Advise patients to take posaconazole delayed-release tablets with food.
Advise patients that posaconazole delayed-release tablets must be swallowed whole and not divided, crushed, or chewed.
Instruct patients that if they miss a dose, they should take it as soon as they remember. If they do not remember until it is within 12 hours of the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not double their next dose or
take more than the prescribed dose.
Drug Interactions
Advise patients to inform their physician immediately if they:
- •
- develop severe diarrhea or vomiting.
- •
- are currently taking drugs that are known to prolong the QTc interval and are metabolized through CYP3A4.
- •
- are currently taking a cyclosporine or tacrolimus, or they notice swelling in an arm or leg or shortness of breath.
- •
- are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of posaconazole.
Serious and Potentially Serious Adverse Reactions
Advise patients to inform their physician immediately if they:
- •
- notice a change in heart rate or heart rhythm, or have a heart condition or circulatory disease. Posaconazole can be administered with caution to patients with potentially proarrhythmic conditions.
- •
- are pregnant, plan to become pregnant, or are nursing.
- •
- have liver disease or develop itching, nausea or vomiting, their eyes or skin turn yellow, they feel more tired than usual or feel like they have the flu.
- •
- have ever had an allergic reaction to other antifungal medicines such as ketoconazole, fluconazole, itraconazole, or voriconazole.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
AET Laboratories Private Limited
Telangana state, 502319,
India
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland - 21202
United States
Distributed By:
MAJOR® PHARMACEUTICALS
Livonia, MI 48152
Refer to package label for Distributor's NDC Number
November 2021
The trademarks referenced herein are owned by their respective companies.
All rights reserved.
-
PATIENT PACKAGE INSERT
Patient Information
Posaconazole (poe sa kon a zole) delayed-release tablets
What is posaconazole delayed-release tablets?
Posaconazole delayed-release tablets are prescription medicines used in adults and children 13 years of age an older to help prevent fungal infections that can spread throughout your body (invasive fungal infections). These infections are caused by fungi called Aspergillus or Candida. Posaconazole delayed-release tablets are used in people who have an increased chance of getting these infections due to a weak immune system. These include people who have had a hematopoietic stem cell transplantation (bone marrow transplant) with graft versus host disease or those with a low white blood cell count due to chemotherapy for blood cancers (hematologic malignancy).
It is not known if posaconazole delayed-release tablets are safe and effective in children under 2 years of age.Who should not take posaconazole delayed-release tablets?
Do not take posaconazole delayed-release tablets if you:
- •
- are allergic to posaconazole, any of the ingredients in posaconazole delayed-release tablets, or other azole antifungal medicines. See the end of this leaflet for a complete list of ingredients in posaconazole delayed-release tablets.
- •
- are taking any of the following medicines:
o sirolimus
o pimozide
o quinidine
o certain statin medicines that lower cholesterol (atorvastatin, lovastatin, simvastatin)
o ergot alkaloids (ergotamine, dihydroergotamine)
Ask your healthcare provider or pharmacist if you are not sure if you are taking any of these medicines.
Do not start taking a new medicine without talking to your healthcare provider or pharmacist.What should I tell my healthcare provider before taking posaconazole delayed-release tablets?
Before you take posaconazole delayed-release tablets, tell your healthcare provider if you:
- •
- are taking certain medicines that lower your immune system like cyclosporine or tacrolimus.
- •
- are taking certain drugs for HIV infection, such as ritonavir, atazanavir, efavirenz, or fosamprenavir. Efavirenz and fosamprenavir can cause a decrease in the posaconazole delayed-release tablets levels in your body. Efavirenz and fosamprenavir should not be taken with posaconazole delayed-release tablets.
- •
- are taking midazolam, a hypnotic and sedative medicine.
- •
- are taking vincristine, vinblastine and other "vinca alkaloids" (medicines used to treat cancer).
- •
- have or had liver problems.
- •
- have or had kidney problems.
- •
- have or had an abnormal heart rate or rhythm, heart problems, or blood circulation problems.
- •
- are pregnant or plan to become pregnant. It is not known if posaconazole delayed-release tablets will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if posaconazole passes into your breast milk. You and your healthcare provider should decide if you will take posaconazole delayed-release tablets or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Posaconazole delayed-release tablets can affect the way other medicines work, and other medicines can affect the way posaconazole delayed-release tablets work and can cause serious side effects.
Especially tell your healthcare provider if you take:
$$UnOrderedlist
rifabutin or phenytoin. If you are taking these medicines, you should not take posaconazole delayed-release tablets.
$$EndUnOrderedlist
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them with you to show your healthcare provider or pharmacist when you get a new medicine.How will I take posaconazole delayed-release tablets?
- •
- Do not switch between posaconazole oral suspension and Posaconazole delayed-release tablets or posaconazole powdermix for delayed-release oral suspension.
- •
- Take posaconazole delayed-release tablets exactly as your healthcare provider tells you to take them.
- •
- Your healthcare provider will tell you how many posaconazole delayed-release tablets to take and when to take it.
- •
- Take posaconazole delayed-release tablets for as long as your healthcare provider tells you to take it.
- •
- If you take too many posaconazole delayed-release tablets, call your healthcare provider or go to the nearest hospital emergency room right away.
- •
- Take posaconazole delayed-release tablets with or without food.
- •
- Take posaconazole delayed-release tablets whole. Do not break, crush, or chew posaconazole delayed-release tablets before swallowing. If you cannot swallow posaconazole delayed-release tablets whole, tell your healthcare provider. You may need a different medicine.
- •
- If you miss a dose, take it as soon as you remember and then take your next scheduled dose at its regular time. If it is within 12 hours of your next dose, do not take the missed dose. Skip the missed dose and go back to your regular schedule. Do not double your next dose or take more than your prescribed dose.
Follow the instructions from your healthcare provider on how many posaconazole delayed-release tablets you should take and when to take them.
What are the possible side effects of posaconazole delayed-release tablets?
Posaconazole delayed-release tablets may cause serious side effects, including:
- •
- drug interactions with cyclosporine or tacrolimus. If you take posaconazole delayed-release tablets with cyclosporine or tacrolimus, your blood levels of cyclosporine or tacrolimus may increase. Serious side effects can happen in your kidney or brain if you have high levels of cyclosporine or tacrolimus in your blood. Your healthcare provider should do blood tests to check your levels of cyclosporine or tacrolimus if you are taking these medicines while taking posaconazole delayed-release tablets. Tell your healthcare provider right away if you have swelling in your arm or leg or shortness of breath.
- •
- problems with the electrical system of your heart (arrhythmias and QTc prolongation). Certain medicines used to treat fungus called azoles, including posaconazole, the active ingredient in posaconazole delayed-release tablets, may cause heart rhythm problems. People who have certain heart problems or who take certain medicines have a higher chance for this problem. Tell your healthcare provider right away if your heartbeat becomes fast or irregular.
- •
- liver problems. Some people who also have other serious medical problems may have severe liver problems that may lead to death, especially if you take certain doses of posaconazole delayed-release tablets. Your healthcare provider should do blood tests to check your liver while you are taking posaconazole delayed-release tablets. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
o itchy skin
o nausea or vomiting
o yellowing of your eyes
o flu-like symptoms
o feeling very tired
- •
- increased amounts of midazolam in your blood. If you take posaconazole delayed-release tablets with midazolam, posaconazole delayed-release tablets increase the amount of midazolam in your blood. This can make your sleepiness last longer. Your healthcare provider should check you closely for side effects if you take midazolam with posaconazole delayed-release tablets.
The most common side effects of posaconazole delayed-release tablets include:
- •
- diarrhea
- •
- nausea
- •
- fever
- •
- vomiting
- •
- headache
- •
- coughing
- •
- low potassium levels in the blood
If you take posaconazole delayed-release tablets, tell your healthcare provider right away if you have diarrhea or vomiting.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of posaconazole delayed-release tablets. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store posaconazole delayed-release tablets?
- •
- Store posaconazole delayed-release tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Safely throw away medicine that is out of date or no longer needed.
Keep posaconazole delayed-release tablets and all medicines out of the reach of children.
General information about the safe and effective use of posaconazole delayed-release tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use posaconazole delayed-release tablets for a condition for which it was not prescribed. Do not give posaconazole delayed-release tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about posaconazole delayed-release tablets that is written for health professionals.What are the ingredients in posaconazole delayed-release tablets?
Active ingredient: posaconazole
Inactive ingredients: partially neutralized methacrylic acid and ethyl acrylate copolymer, triethyl citrate, xylitol, hydroxypropyl cellulose, propyl gallate, cellulose, microcrystalline, silica, colloidal anhydrous, croscarmellose sodium, sodium stearyl fumarate and Opadry® II Yellow (consists of the following ingredients: polyvinyl alcohol partially hydrolyzed, macrogol, polyethylene glycol, titanium dioxide, talc, and iron oxide yellow).
Additional Pediatric Use information is approved for Merck Sharp & Dohme Corp.'s NOXAFIL (posaconazole) delayed-release tablets. However, due to Merck Sharp & Dohme Corp.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information.Manufactured by:
AET Laboratories Private Limited
Telangana state, 502319,
India
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland - 21202
United StatesDistributed By:
MAJOR® PHARMACEUTICALS
Livonia, MI 48152
Distributed By:
Cardinal Health
Dublin, OH 43017
L58544500823
Refer to package label for Distributor's NDC Number
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised:. November 2021
ID#: 40284 - Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
POSACONAZOLE
posaconazole tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55154-4322(NDC:0904-7149) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POSACONAZOLE (UNII: 6TK1G07BHZ) (POSACONAZOLE - UNII:6TK1G07BHZ) POSACONAZOLE 100 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYL GALLATE (UNII: 8D4SNN7V92) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color YELLOW Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 100P Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55154-4322-0 10 in 1 BAG 08/01/2023 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213454 02/03/2021 Labeler - Cardinal Health 107, LLC (118546603)