Label: ASPIRIN AND EXTENDED-RELEASE DIPYRIDAMOLE capsule, extended release
- NDC Code(s): 42571-274-60
- Packager: Micro Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ASPIRIN and EXTENDED-RELEASE DIPYRIDAMOLE CAPSULES safely and effectively. See full prescribing information for ASPIRIN and EXTENDED-RELEASE DIPYRIDAMOLE CAPSULES.
ASPIRIN and extended-release DIPYRIDAMOLE capsules, for oral use
Initial U.S. Approval: 1999INDICATIONS AND USAGE
- Aspirin and extended-release dipyridamole capsule is a combination of aspirin and dipyridamole, antiplatelet agents, indicated to reduce the risk of stroke in patients who have had transient ischemia of the brain or completed ischemic stroke due to thrombosis ( 1)
DOSAGE AND ADMINISTRATION
- One capsule twice daily (morning and evening) with or without food (2)
- In case of intolerable headaches during initial treatment, switch to one capsule at bedtime and low-dose aspirin in the morning; resume BID dosing within one week (2.1)
- Do not chew capsule (2)
- Not interchangeable with the individual components of aspirin and dipyridamole tablets(2)
- Dispense in this unit-of-use container (16)
DOSAGE FORMS AND STRENGTHS
- Capsule: 25 mg aspirin/200 mg extended-release dipyridamole (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Aspirin and extended-release dipyridamole capsules increases the risk of bleeding ( 5.1)
- Avoid use in patients with severe hepatic or renal insufficiency ( 5.2, 5.3)
- Interrupt aspirin and extended-release dipyridamole capsules 48 hours before using intravenous dipyridamole or other adenosinergic agents for stress testing ( 5.6, 7.1)
ADVERSE REACTIONS
- The most frequently reported adverse reactions (>10% and greater than placebo) were headache, dyspepsia, abdominal pain, nausea, and diarrhea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Micro Labs USA, Inc. at 1-855-839-8195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Alternative Regimen in Case of Intolerable Headaches
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Allergy
4.3 Reye Syndrome
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Bleeding
5.2 Renal Failure
5.3 Hepatic Insufficiency
5.4 Coronary Artery Disease
5.5 Hypotension
5.6 Stress Testing with Intravenous Dipyridamole and Other Adenosinergic Agents
5.7 General
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Drug Interaction Study Information Obtained From Literature
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Severe Hepatic or Severe Renal Dysfunction
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Aspirin and extended-release dipyridamole capsule is not interchangeable with the individual components of aspirin and dipyridamole tablets.
The recommended dose of aspirin and extended-release dipyridamole capsules is one capsule given orally twice daily, one in the morning and one in the evening. Swallow capsules whole without chewing. Aspirin and extended-release dipyridamole capsules can be administered with or without food.
2.1 Alternative Regimen in Case of Intolerable Headaches
In the event of intolerable headaches during initial treatment, switch to one capsule at bedtime and low-dose aspirin in the morning. Because there are no outcome data with this regimen and headaches become less of a problem as treatment continues, patients should return to the usual regimen as soon as possible, usually within one week.
-
3 DOSAGE FORMS AND STRENGTHS
Aspirin and extended-release Dipyridamole 25 mg/200 mg capsules are available as a hard gelatin capsule, with red opaque colored cap and ivory opaque colored body, size ‘0 Xel’ hard gelatin capsule imprinted with ‘M’ on cap and ‘25’ on body in black ink, containing yellow colored extended-release pellets of Dipyridamole and a yellow colored immediate release pellets of Aspirin.
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Aspirin and extended-release dipyridamole capsule is contraindicated in patients with known hypersensitivity to any of the product components.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Bleeding
Aspirin and extended-release dipyridamole increases the risk of bleeding. Risk factors for bleeding include the use of other drugs that increase the risk of bleeding (e.g., anticoagulants, antiplatelet agents, heparin, anagrelide, fibrinolytic therapy, and chronic use of NSAIDs) [ see Drug Interactions (7.1)].
Intracranial Hemorrhage
In European Stroke Prevention Study-2 (ESPS2), the annualized event rate for intracranial hemorrhage was 0.39%/year in the aspirin and extended-release dipyridamole capsules group, 0.26%/year in the extended-release dipyridamole (ER-DP) group, 0.24%/year in the aspirin (ASA) group, and 0.29%/year in the placebo groups.
Gastrointestinal (GI) Side Effects
GI side effects include stomach pain, heartburn, nausea, vomiting, and gross GI bleeding. Although minor upper GI symptoms, such as dyspepsia, are common and can occur anytime during therapy, physicians should remain alert for signs of ulceration and bleeding, even in the absence of previous GI symptoms. Inform patients about the signs and symptoms of GI side effects and what steps to take if they occur .
In ESPS2, the annualized event rate for gastrointestinal bleeding was 2.97%/year in the aspirin and extended-release dipyridamole capsules group, 1.58%/year in the extended-release dipyridamole group, 2.06%/year in the aspirin group, and 1.40%/year in the placebo groups.
Peptic Ulcer Disease
Avoid using aspirin in patients with a history of active peptic ulcer disease, which can cause gastric mucosal irritation and bleeding.
Alcohol Warning
Because aspirin and extended-release dipyridamole capsules contains aspirin, counsel patients who consume three or more alcoholic drinks every day about the bleeding risks involved with chronic, heavy alcohol use while taking aspirin.
5.2 Renal Failure
Avoid aspirin in patients with severe renal failure (glomerular filtration rate less than 10 mL/minute) [ see Use in Specific Populations ( 8.6) and Clinical Pharmacology (12.3)].
5.3 Hepatic Insufficiency
Elevations of hepatic enzymes and hepatic failure have been reported in association with dipyridamole administration [ see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
5.4 Coronary Artery Disease
Dipyridamole has a vasodilatory effect. Chest pain may be precipitated or aggravated in patients with underlying coronary artery disease who are receiving dipyridamole.
For stroke or TIA patients for whom aspirin is indicated to prevent recurrent myocardial infarction (MI) or angina pectoris, the aspirin in this product may not provide adequate treatment for the cardiac indications.
5.5 Hypotension
Dipyridamole produces peripheral vasodilation, which can exacerbate pre-existing hypotension.
5.6 Stress Testing with Intravenous Dipyridamole and Other Adenosinergic Agents
Clinical experience suggests that patients being treated with aspirin and extended-release dipyridamole capsules who also require pharmacological stress testing with intravenous dipyridamole or other adenosinergic agents (e.g. adenosine, regadenoson) should interrupt aspirin and extended-release dipyridamole capsules for 48 hours prior to stress testing [see Drug Interactions (7.1)].
Intake of aspirin and extended-release dipyridamole capsules within 48 hours prior to stress testing with intravenous dipyridamole or other adenosinergic agents may increase the risk for cardiovascular side effects of these agents and may impair the sensitivity of the test.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling:
- Hypersensitivity [ see Contraindications (4.1)]
- Allergy [ see Contraindications (4.2)]
- Risk of Bleeding [ see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The efficacy and safety of aspirin and extended-release dipyridamole was established in the European Stroke Prevention Study-2 (ESPS2). ESPS2 was a double-blind, placebo-controlled study that evaluated 6602 patients over the age of 18 years who had a previous ischemic stroke or transient ischemic attack within ninety days prior to entry. Patients were randomized to either aspirin and extended-release dipyridamole capsules, aspirin, ER-DP, or placebo [ see Clinical Studies (14)]; primary endpoints included stroke (fatal or nonfatal) and death from all causes.
This 24-month, multicenter, double-blind, randomized study (ESPS2) was conducted to compare the efficacy and safety of aspirin and extended-release dipyridamole capsules with placebo, extended-release dipyridamole alone and aspirin alone. The study was conducted in a total of 6602 male and female patients who had experienced a previous ischemic stroke or transient ischemia of the brain within three months prior to randomization.
Table 1 presents the annualized event rate for adverse events that occurred in 1%/year or more of patients treated with aspirin and extended-release dipyridamole capsules where the incidence was also at least 1%/year greater than in those patients treated with placebo. There is no clear benefit of the dipyridamole/aspirin combination over aspirin with respect to safety.
Table 1 Incidence of Adverse Events in ESPS2 a
Individual Treatment Group
Body System/Preferred Term
Aspirin And Extended-Release Dipyridamole
n (%/year) b
ER-DP Alone
n (%/year) b
ASA Alone
n (%/year) b
Placebo
n (%/year) b
Total Number of Patients
1650
1654
1649
1649
Central and Peripheral Nervous System Disorders
Headache
647(28.25)
634 (27.91)
558 (22.10)
543 (22.29)
Gastrointestinal System Disorders
Dyspepsia
303 (13.23)
288 (12.68)
299 (11.84)
275 (11.29)
Abdominal Pain
289 (12.62)
255 (11.22)
262 (10.38)
239 (9.81)
Nausea
264 (11.53)
254 (11.18)
210 (8.32)
232 (9.53)
Diarrhea
210 (9.17)
257 (11.31)
112 (4.44)
161 (6.61)
Vomiting
138 (6.03)
129 (5.68)
101 (4.00)
118 (4.84)
Platelet, Bleeding and Clotting Disorders
Hemorrhage NOS
52 (2.27)
24 (1.06)
46 (1.82)
24 (0.99)
aReported by ≥1%/year of patients during aspirin and extended-release dipyridamole capsules treatment where the incidence was at least 1%/year greater than in those treated with placebo.
bAnnual event rate per 100 pt-years = 100* number of subjects with event/subject-years. Subject-years is defined as cumulative number of days on treatment divided by 365.25.
Note: ER-DP = extended-release dipyridamole 200 mg; ASA = aspirin 25 mg. The dosage regimen for all treatment groups is BID.
NOS = not otherwise specified.
Discontinuation due to adverse events in ESPS2 was 25% for aspirin and extended-release dipyridamole, 25% for extended-release dipyridamole, 19% for aspirin, and 21% for placebo (refer to Table 2).
Table 2 Incidence of Adverse Events that Led to the Discontinuation of Treatment a
Treatment Groups
Aspirin And Extended-Release Dipyridamole
n (%/year) b
ER-DP n (%/year) b
ASA n (%/year) b
Placebo n (%/year) b
Total Number of Patients
1650
1654
1649
1649
Patients with at least one Adverse Event that led to treatment discontinuation
417 (18.21)
419 (18.44)
318 (12.59)
352 (14.45)
Headache
165 (7.20)
166 (7.31)
57 (2.26)
69 (2.83)
Nausea
91 (3.97)
95 (4.18)
51 (2.02)
53 (2.18)
Abdominal Pain
74 (3.23)
64 (2.82)
56 (2.22)
52 (2.13)
Vomiting
53 (2.31)
52 (2.29)
28 (1.11)
24 (0.99)
aReported by ≥1%/year of patients during aspirin and extended-release dipyridamole capsules treatment where the incidence was at least 1%/year greater than in those treated with placebo.
bAnnual event rate per 100 pt-years = 100* number of subjects with event/subject-years. Subject-years is defined as cumulative number of days on treatment divided by 365.25.
Note: ER-DP = extended-release dipyridamole 200 mg; ASA = aspirin 25 mg. The dosage regimen for all treatment groups is BID.
Headache was most notable in the first month of treatment.
6.2 Post-Marketing Experience
The following is a list of additional adverse reactions that have been reported either in the literature or are from post-marketing spontaneous reports for either dipyridamole or aspirin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to aspirin and extended-release dipyridamole.
Body as a Whole: Hypothermia, chest pain, allergic reaction, syncope
Cardiovascular: Angina pectoris, hypotension
Central Nervous System: Cerebral edema, dizziness, cerebral hemorrhage, intracranial hemorrhage, subarachnoid hemorrhage
Fluid and Electrolyte: Hyperkalemia, metabolic acidosis, respiratory alkalosis, hypokalemia
Gastrointestinal: Pancreatitis, Reye syndrome, hematemesis, gastritis, ulceration and perforation, hemorrhage rectum, melena, GI hemorrhage
Hearing and Vestibular Disorders: Hearing loss
Heart Rate and Rhythm Disorders: Tachycardia, palpitation
Immune System Disorders: Hypersensitivity, acute anaphylaxis, laryngeal edema
Liver and Biliary System Disorders: Hepatitis, hepatic failure, cholelithiasis, jaundice, hepatic function abnormal
Musculoskeletal: Rhabdomyolysis, myalgia
Metabolic and Nutritional Disorders: Hypoglycemia, dehydration
Platelet, Bleeding and Clotting Disorders: Prolongation of the prothrombin time, disseminated intravascular coagulation, coagulopathy, thrombocytopenia, hematoma, gingival bleeding, epistaxis, purpura
Psychiatric Disorders: Confusion, agitation
Respiratory: Tachypnea, dyspnea, hemoptysis
Skin and Appendages Disorders: Rash, alopecia, angioedema, Stevens-Johnson syndrome, skin hemorrhages such as bruising, ecchymosis, and hematoma, pruritus, urticaria, and drug reaction with eosinophilia and systemic symptoms (DRESS)
Urogenital: Interstitial nephritis, papillary necrosis, proteinuria, renal insufficiency and failure, hematuria
Vascular (Extracardiac) Disorders: Allergic vasculitis, flushing
Other Adverse Events: Anorexia, aplastic anemia, migraine, pancytopenia, thrombocytosis.
-
7 DRUG INTERACTIONS
7.1 Drug Interaction Study Information Obtained From Literature
Adenosinergic agents (e.g. adenosine, regadenoson)
Dipyridamole has been reported to increase the plasma levels and cardiovascular effects of adenosine. Adjustment of adenosine dosage may be necessary. Dipyridamole also increases the cardiovascular effects of regadenoson, an adenosine A 2A-receptor agonist. The potential risk of cardiovascular side effects with intravenous adenosinergic agents may be increased during the testing period when dipyridamole is not held 48 hours prior to stress testing.
Angiotensin Converting Enzyme (ACE) Inhibitors
Because of the indirect effect of aspirin on the renin-angiotensin conversion pathway, the hyponatremic and hypotensive effects of ACE inhibitors may be diminished by concomitant administration of aspirin.
Acetazolamide
Concurrent use of aspirin and acetazolamide can lead to high serum concentrations of acetazolamide (and toxicity) due to competition at the renal tubule for secretion.
Anticoagulants and Antiplatelets
Patients taking aspirin and extended-release dipyridamole capsules in combination with anticoagulants, antiplatelets, or any substance impacting coagulation are at increased risk for bleeding. Aspirin can displace warfarin from protein binding sites, leading to prolongation of both the prothrombin time and the bleeding time. Aspirin can increase the anticoagulant activity of heparin, increasing bleeding risk.
Anagrelide
Patients taking aspirin in combination with anagrelide are at an increased risk of bleeding.
Anticonvulsants
Salicylic acid can displace protein-bound phenytoin and valproic acid, leading to a decrease in the total concentration of phenytoin and an increase in serum valproic acid levels.
Beta Blockers
The hypotensive effects of beta blockers may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow and salt and fluid retention.
Cholinesterase Inhibitors
Dipyridamole may counteract the anticholinesterase effect of cholinesterase inhibitors, thereby potentially aggravating myasthenia gravis.
Diuretics
The effectiveness of diuretics in patients with underlying renal or cardiovascular disease may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow and salt and fluid retention.
Methotrexate
Salicylate can inhibit renal clearance of methotrexate, leading to bone marrow toxicity, especially in the elderly or renal impaired.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
The concurrent use of aspirin with other NSAIDs may increase bleeding or lead to decreased renal function.
Oral Hypoglycemics
Moderate doses of aspirin may increase the effectiveness of oral hypoglycemic drugs, leading to hypoglycemia.
Uricosuric Agents (probenecid and sulfinpyrazone)
Salicylates antagonize the uricosuric action of uricosuric agents.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published studies and postmarketing experience with aspirin and extended-release dipyridamole capsules use during pregnancy have not identified a clear association between aspirin and extended-release dipyridamole capsules use and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Aspirin and extended-release dipyridamole capsule contains low-dose aspirin which is an NSAID (see Clinical Considerations). In animal reproduction studies, there were adverse developmental effects with administration of aspirin in rats and rabbits at doses about 66 and 44 times, respectively, the human exposure at the maximum recommended daily dose of aspirin-dipyridamole. Reproduction studies with dipyridamole in mice, rabbits, and rats have revealed no evidence of harm to the fetus up to doses about 25 times the maximum recommended daily human dose of aspirin-dipyridamole. Nonclinical data are suggestive of a possible potentiation of aspirin-related fetal toxicity when combined with dipyridamole (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 and 15 to 20%, respectively.
Clinical Considerations
Labor and Delivery
Aspirin and extended-release dipyridamole capsules, which contains dipyridamole and low-dose aspirin, increases the risk for bleeding [see Warnings and Precautions (5.1)]. Maternal use of high-dose aspirin can result in excessive blood loss at delivery, prolonged gestation, prolonged labor, intracranial hemorrhage in premature infants, low birth weight, stillbirth, and neonatal death.
Data
Human Data
Published data from clinical trials, observational studies, case series, and case reports over several decades have not identified a clear association between aspirin dipyridamole use in pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes. However, these studies cannot definitively establish the absence of any aspirin-dipyridamole associated risks. Methodological limitations of these studies include variability in the timing and dose of drug exposure (e.g., most exposures occurred beyond the first trimester) and the small sample sizes of individual studies.
Animal Data
Aspirin has been shown to be teratogenic in rats (spina bifida, exencephaly, microphthalmia and coelosomia) and rabbits (congested fetuses, agenesis of skull and upper jaw, generalized edema with malformation of the head, and diaphanous skin) at oral doses of 330 mg/kg/day and 110 mg/kg/day, respectively. These doses, which also resulted in a high resorption rate in rats (63% of implantations versus 5% in controls), are, on a mg/m2 basis, about 66 and 44 times, respectively, the dose of aspirin contained in the maximum recommended daily human dose of aspirin-dipyridamole. Reproduction studies with dipyridamole have been performed in mice, rabbits and rats at oral doses of up to 125 mg/kg, 40 mg/kg, and 1000 mg/kg, respectively (about 1½, 2, and 25 times the maximum recommended daily human oral dose, respectively, on a mg/m 2 basis) and have revealed no evidence of harm to the fetus due to dipyridamole. When 330 mg aspirin/kg/day was combined with 75 mg dipyridamole/kg/day in the rat at doses about 66 and 2 times, respectively, the maximum recommended daily human dose, the resorption rate approached 100%.
8.2 Lactation
Risk Summary
Based on data from a clinical lactation study in breastfeeding women taking low-dose aspirin, the metabolite salicylic acid is present in human milk in low levels (see Data). Dipyridamole is also present in human milk. There is no information on the effects of aspirin and extended-release dipyridamole capsules or dipyridamole on the breastfed infant or on milk production. There is insufficient information to determine the effects of aspirin on the breastfed infant and no information on the effects of aspirin on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for aspirin and extended-release dipyridamole capsules and any potential adverse effects on the breastfed child from aspirin and extended-release dipyridamole capsules or from the underlying maternal condition.
Data
A published clinical study involved six exclusively breastfeeding women at 1 to 8 months postpartum who were taking 81 mg aspirin daily. Milk samples were collected at steady state, at 0, 1, 2, 4, 8, 12, and 24 hours after taking a dose of aspirin. Aspirin was undetectable in human milk. Salicylic acid was present in milk at low levels (average concentration of 24 ng/mL). Based on an average milk consumption of 150 mL/kg/day, the calculated relative infant dose was 0.4%. No adverse effects on the breastfed infants were noted.
8.4 Pediatric Use
Safety and effectiveness of aspirin and extended-release dipyridamole in pediatric patients have not been studied. Because of the aspirin component, use of this product in the pediatric population is not recommended [ see Contraindications (4.3)].
8.5 Geriatric Use
Of the total number of subjects in ESPS2, 61% were 65 and over, while 27% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out [ see Clinical Pharmacology (12.3)].
8.6 Patients with Severe Hepatic or Severe Renal Dysfunction
Aspirin and extended-release dipyridamole has not been studied in patients with hepatic or renal impairment. Avoid using aspirin containing products, such as aspirin and extended-release dipyridamole in patients with severe hepatic or severe renal (glomerular filtration rate <10 mL/min) dysfunction [ see Warnings and Precautions (5.2, 5.3) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Because of the dose ratio of dipyridamole to aspirin, overdosage of aspirin and extended-release dipyridamole capsule is likely to be dominated by signs and symptoms of dipyridamole overdose. In case of real or suspected overdose, seek medical attention or contact a Poison Control Center immediately. Careful medical management is essential.
Based upon the known hemodynamic effects of dipyridamole, symptoms such as warm feeling, flushes, sweating, restlessness, feeling of weakness, and dizziness may occur. A drop in blood pressure and tachycardia might also be observed.
Salicylate toxicity may result from acute ingestion (overdose) or chronic intoxication. Severity of aspirin intoxication is determined by measuring the blood salicylate level. The early signs of salicylic overdose (salicylism), including tinnitus (ringing in the ears), occur at plasma concentrations approaching 200 mcg/mL. In severe cases, hyperthermia and hypovolemia are the major immediate threats to life. Plasma concentrations of aspirin above 300 mcg/mL are clearly toxic. Severe toxic effects are associated with levels above 400 mcg/mL. A single lethal dose of aspirin in adults is not known with certainty but death may be expected at 30 g.
Treatment of overdose consists primarily of supporting vital functions, increasing drug elimination, and correcting acid-base disturbances. Consider gastric emptying and/or lavage as soon as possible after ingestion, even if the patient has vomited spontaneously. After lavage and/or emesis, administration of activated charcoal as a slurry may be beneficial if less than 3 hours have passed since ingestion. Charcoal absorption should not be employed prior to emesis and lavage. Follow acid-base status closely with serial blood gas and serum pH measurements. Maintain fluid and electrolyte balance. Administer replacement fluid intravenously and augment with correction of acidosis. Treatment may require the use of a vasopressor. Infusion of glucose may be required to control hypoglycemia.
Administration of xanthine derivatives (e.g., aminophylline) may reverse the vasodilatory effects of dipyridamole overdose. Plasma electrolytes and pH should be monitored serially to promote alkaline diuresis of salicylate if renal function is normal. In patients with renal insufficiency or in cases of life-threatening intoxication, dialysis is usually required to treat salicylic overdose; however, since dipyridamole is highly protein bound, dialysis is not likely to remove dipyridamole. Exchange transfusion may be indicated in infants and young children.
-
11 DESCRIPTION
Aspirin and extended-release dipyridamole capsule is a combination antiplatelet agent intended for oral administration. Each hard gelatin capsule contains 200 mg dipyridamole in an extended-release form and 25 mg aspirin, as an immediate-release. In addition, each capsule contains the following inactive ingredients: caprylic/capric mono/diglycerides, D&C yellow #10 Aluminum Lake, FD&C blue #1/brilliant blue FCF Aluminum Lake, glyceryl monostearate, hypromellose, methacrylic acid and methacrylate copolymer, microcrystalline cellulose, povidone K30, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, talc, tartaric acid, titanium dioxide, triacetin and triethyl citrate.
Each capsule shell contains gelatin, ferric oxide red, ferric oxide yellow, titanium dioxide and water.
Black imprint ink composition contains ferrosoferric oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
Dipyridamole USP
Dipyridamole USP is an antiplatelet agent chemically described as 2,2',2'',2'''-[(4,8-Dipiperidinopyrimido[5,4- d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula:
Dipyridamole is an odorless yellow crystalline substance, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and is practically insoluble in water.
Aspirin USP
The antiplatelet agent aspirin (acetylsalicylic acid) is chemically known as benzoic acid, 2-(acetyloxy)-, and has the following structural formula:
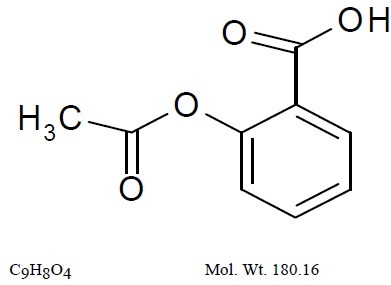
Aspirin is an odorless white needle-like crystalline or powdery substance. When exposed to moisture, aspirin hydrolyzes into salicylic and acetic acids, and gives off a vinegary odor. It is highly lipid soluble and slightly soluble in water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The antithrombotic action of aspirin and extended-release dipyridamole capsule is the result of the additive antiplatelet effects of dipyridamole and aspirin.
Dipyridamole
Dipyridamole inhibits the uptake of adenosine into platelets, endothelial cells and erythrocytes in vitro and in vivo; the inhibition occurs in a dose-dependent manner at therapeutic concentrations (0.5 to 1.9 mcg/mL). This inhibition results in an increase in local concentrations of adenosine which acts on the platelet A 2-receptor thereby stimulating platelet adenylate cyclase and increasing platelet cyclic-3',5'-adenosine monophosphate (cAMP) levels. Via this mechanism, platelet aggregation is inhibited in response to various stimuli such as platelet activating factor (PAF), collagen and adenosine diphosphate (ADP).
Dipyridamole inhibits phosphodiesterase (PDE) in various tissues. While the inhibition of cAMP-PDE is weak, therapeutic levels of dipyridamole inhibit cyclic-3',5'guanosine monophosphate-PDE (cGMP-PDE), thereby augmenting the increase in cGMP produced by EDRF (endothelium-derived relaxing factor, now identified as nitric oxide).
Aspirin
Aspirin inhibits platelet aggregation by irreversible inhibition of platelet cyclooxygenase and thus inhibits the generation of thromboxane A 2, a powerful inducer of platelet aggregation and vasoconstriction.
12.2 Pharmacodynamics
The effect of either agent on the other's inhibition of platelet reactivity has not been evaluated.
12.3 Pharmacokinetics
There are no significant interactions between aspirin and dipyridamole. The kinetics of the components are unchanged by their co-administration as aspirin and extended-release dipyridamole capsules.
Absorption
Dipyridamole:
Peak plasma levels of dipyridamole are achieved 2 hours (range 1 to 6 hours) after administration of a daily dose of 400 mg aspirin and extended-release dipyridamole capsules (given as 200 mg BID). The peak plasma concentration at steady-state is 1.98 mcg/mL (1.01 to 3.99 mcg/mL) and the steady-state trough concentration is 0.53 mcg/mL (0.18 to 1.01 mcg/mL).
Aspirin:
Peak plasma levels of aspirin are achieved 0.63 hours (0.5 to 1 hour) after administration of a 50 mg aspirin daily dose from aspirin and extended-release dipyridamole capsules (given as 25 mg BID). The peak plasma concentration at steady-state is 319 ng/mL (175 to 463 ng/mL). Aspirin undergoes moderate hydrolysis to salicylic acid in the liver and the gastrointestinal wall, with 50% to 75% of an administered dose reaching the systemic circulation as intact aspirin.
Effect of Food
Dipyridamole:
When aspirin and extended-release dipyridamole capsules were taken with a high fat meal, dipyridamole peak plasma levels (C max) and total absorption (AUC) were decreased at steady-state by 20 to 30% compared to fasting. Due to the similar degree of inhibition of adenosine uptake at these plasma concentrations, this food effect is not considered clinically relevant.
Aspirin:
When aspirin and extended-release dipyridamole capsules were taken with a high fat meal, there was no difference for aspirin in AUC at steady-state, and the approximately 50% decrease in C max was not considered clinically relevant based on a similar degree of cyclooxygenase inhibition comparing the fed and fasted state.
Distribution
Dipyridamole:
Dipyridamole is highly lipophilic (log P=3.71, pH=7); however, it has been shown that the drug does not cross the blood-brain barrier to any significant extent in animals. The steady-state volume of distribution of dipyridamole is about 92 L. Approximately 99% of dipyridamole is bound to plasma proteins, predominantly to alpha 1-acid glycoprotein and albumin.
Aspirin:
Aspirin is poorly bound to plasma proteins and its apparent volume of distribution is low (10 L). Its metabolite, salicylic acid, is highly bound to plasma proteins, but its binding is concentration-dependent (nonlinear). At low concentrations (<100 mcg/mL), approximately 90% of salicylic acid is bound to albumin. Salicylic acid is widely distributed to all tissues and fluids in the body, including the central nervous system, breast milk, and fetal tissues. Early signs of salicylate overdose (salicylism), including tinnitus (ringing in the ears), occur at plasma concentrations approximating 200 mcg/mL [see Overdosage (10)] .
Metabolism and Elimination
Dipyridamole:
Dipyridamole is metabolized in the liver, primarily by conjugation with glucuronic acid, of which monoglucuronide which has low pharmacodynamic activity is the primary metabolite. In plasma, about 80% of the total amount is present as parent compound and 20% as monoglucuronide. Most of the glucuronide metabolite (about 95%) is excreted via bile into the feces, with some evidence of enterohepatic circulation. Renal excretion of parent compound is negligible and urinary excretion of the glucuronide metabolite is low (about 5%). With intravenous (i.v.) treatment of dipyridamole, a triphasic profile is obtained: a rapid alpha phase, with a half-life of about 3.4 minutes, a beta phase, with a half-life of about 39 minutes, (which, together with the alpha phase accounts for about 70% of the total area under the curve, AUC) and a prolonged elimination phase λ z with a half-life of about 15.5 hours. Because of the extended absorption phase of the dipyridamole component, only the terminal phase is apparent from oral treatment with aspirin and extended-release dipyridamole capsules which was 13.6 hours.
Aspirin:
Aspirin is rapidly hydrolyzed in plasma to salicylic acid, with a half-life of 20 minutes. Plasma levels of aspirin are essentially undetectable 2 to 2.5 hours after dosing and peak salicylic acid concentrations occur 1 hour (range: 0.5 to 2 hours) after administration of aspirin. Salicylic acid is primarily conjugated in the liver to form salicyluric acid, a phenolic glucuronide, an acyl glucuronide, and a number of minor metabolites. Salicylate metabolism is saturable and total body clearance decreases at higher serum concentrations due to the limited ability of the liver to form both salicyluric acid and phenolic glucuronide. Following toxic doses (10 to 20 g), the plasma half-life may be increased to over 20 hours.
The elimination of acetylsalicylic acid follows first-order kinetics with aspirin and extended-release dipyridamole capsules and has a half-life of 0.33 hours. The half-life of salicylic acid is 1.71 hours. Both values correspond well with data from the literature at lower doses which state a resultant half-life of approximately 2 to 3 hours. At higher doses, the elimination of salicylic acid follows zero-order kinetics (i.e., the rate of elimination is constant in relation to plasma concentration), with an apparent half-life of 6 hours or higher. Renal excretion of unchanged drug depends upon urinary pH. As urinary pH rises above 6.5, the renal clearance of free salicylate increases from <5% to >80%. Alkalinization of the urine is a key concept in the management of salicylate overdose [see Overdosage (10)] . Following therapeutic doses, about 10% is excreted as salicylic acid and 75% as salicyluric acid, as the phenolic and acyl glucuronides, in urine.
Specific Populations
Geriatric Patients:
Dipyridamole:
In ESPS2 [see Clinical Studies (14)] , plasma concentrations (determined as AUC) of dipyridamole in healthy elderly subjects (>65 years) were about 40% higher than in subjects younger than 55 years receiving treatment with aspirin and extended-release dipyridamole capsules.
Hepatic Dysfunction:
No study has been conducted with aspirin and extended-release dipyridamole capsules in patients with hepatic dysfunction.
Dipyridamole:
In a study conducted with an intravenous formulation of dipyridamole, patients with mild to severe hepatic insufficiency showed no change in plasma concentrations of dipyridamole but showed an increase in the pharmacologically inactive monoglucuronide metabolite. Dipyridamole can be dosed without restriction as long as there is no evidence of hepatic failure.
Aspirin:
Avoid aspirin in patients with severe hepatic insufficiency.
Renal Dysfunction:
Dipyridamole:
In ESPS2 patients [see Clinical Studies (14)] , with creatinine clearances ranging from about 15 mL/min to >100 mL/min, no changes were observed in the pharmacokinetics of dipyridamole or its glucuronide metabolite if data were corrected for differences in age.
Aspirin:
Avoid aspirin in patients with severe renal failure (glomerular filtration rate <10 mL/min).
Drug Interaction Studies
A dedicated drug interaction study was conducted in 60 healthy volunteers to evaluate the effects of omeprazole 80 mg administered once daily on the pharmacokinetics (PK) of dipyridamole and the pharmacodynamics (PD) of acetylsalicylic acid when co-administered with aspirin and extended-release dipyridamole capsules twice daily. Dipyridamole exposure (C max and AUC) at steady-state were similar with or without omeprazole co-administration. The pharmacokinetics of acetylsalicylic acid was not characterized. However, the antiplatelet activity as measured by arachidonic acid induced platelet aggregation was similar between the treatment arms at steady-state.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In studies in which dipyridamole was administered in the feed to mice (up to 111 weeks in males and females) and rats (up to 128 weeks in males and up to 142 weeks in females), there was no evidence of drug-related carcinogenesis. The highest dose administered in these studies (75 mg/kg/day) was, on a mg/m 2 basis, about equivalent to the maximum recommended daily human oral dose (MRHD) in mice and about twice the MRHD in rats.
Combinations of dipyridamole and aspirin (1:5 ratio) tested negative in the Ames test, in vivo chromosome aberration tests (in mice and hamsters), oral micronucleus tests (in mice and hamsters) and oral dominant lethal test (in mice). Aspirin, alone, induced chromosome aberrations in cultured human fibroblasts. Mutagenicity tests of dipyridamole alone with bacterial and mammalian cell systems were negative.
Combinations of dipyridamole and aspirin have not been evaluated for effects on fertility and reproductive performance. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (about 12 times the MRHD on a mg/m 2 basis). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg (more than 30 times the MRHD on a mg/m 2 basis). Aspirin inhibits ovulation in rats.
-
14 CLINICAL STUDIES
ESPS2 (European Stroke Prevention Study-2) was a double-blind, placebo-controlled, 24-month study in which 6602 patients over the age of 18 years had an ischemic stroke (76%) or transient ischemic attack (TIA, 24%) within three months prior to entry. Patients were enrolled in 13 European countries between February 1989 and May 1995 and were randomized to one of four treatment groups: aspirin and extended-release dipyridamole 25 mg/200 mg; extended-release dipyridamole (ER-DP) 200 mg alone; aspirin (ASA) 25 mg alone; or placebo. The mean age in this population was 66.7 years with 58% of them being males. Patients received one capsule twice daily (morning and evening). Efficacy assessments included analyses of stroke (fatal or nonfatal) and death (from all causes) as confirmed by a blinded morbidity and mortality assessment group. There were no differences with regard to efficacy based on age or gender; patients who were older had a trend towards more events.
Stroke Endpoint
Aspirin and extended-release dipyridamole capsules reduced the risk of stroke by 22.1% compared to aspirin 50 mg/day alone (p = 0.008) and reduced the risk of stroke by 24.4% compared to extended-release dipyridamole 400 mg/day alone (p = 0.002) (Table 3). Aspirin and extended-release dipyridamole capsules reduced the risk of stroke by 36.8% compared to placebo (p <0.001).
Table 3 Summary of First Stroke (Fatal or Nonfatal): ESPS2: Intent-to-Treat Population
Total Number of Patients n
Number of Patients With Stroke Within 2 Years n (%)
Kaplan-Meier Estimate of Survival at 2 Years (95% C.I.)
Gehan-Wilcoxon Test P-value
Risk Reduction at 2 Years
Odds Ratio (95% C.I.)
Individual Treatment Group
Aspirin and extended-release dipyridamole
1650
157 (9.5%)
89.9% (88.4%, 91.4%)
-
-
-
ER-DP
1654
211(12.8%)
86.7% (85%, 88.4%)
-
-
-
ASA
1649
206(12.5%)
87.1% (85.4%, 88.7%)
-
-
-
Placebo
1649
250(15.2%)
84.1% (82.2%, 85.9%)
-
-
-
Pairwise Treatment Group Comparisons
Aspirin and extended-release dipyridamole vs. ER-DP
-
-
-
0.002 b
24.4%
0.72 (0.58, 0.90)
Aspirin and extended-release dipyridamole vs. ASA
-
-
-
0.008 b
22.1%
0.74 (0.59, 0.92)
Aspirin and extended-release dipyridamole vs. Placebo
-
-
-
<0.001 b
36.8%
0.59 (0.48, 0.73)
ER-DP vs. Placebo
-
-
-
0.036 a
16.5%
0.82 (0.67, 1.00)
ASA vs. Placebo
-
-
-
0.009 b
18.9%
0.80 (0.66, 0.97)
a0.010 <p-value ≤0.050; bp-value ≤0.010.
Note: ER-DP = extended-release dipyridamole 200 mg; ASA = aspirin 25 mg. The dosage regimen for all treatment groups is BID.
Figure 1 ESPS2: Cumulative Stroke Rate (Fatal or Nonfatal)
Over 24 months of Follow-Up
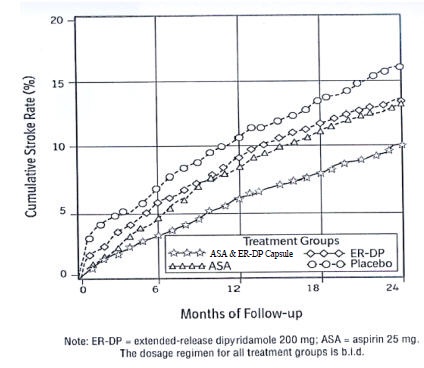
Combined Stroke or Death Endpoint
In ESPS2, aspirin and extended-release dipyridamole capsule reduced the risk of stroke or death by 24.2% compared to placebo.
Aspirin and extended-release dipyridamole capsule reduced the risk of stroke or death by 12.1% compared to aspirin alone and by 10.3% compared to extended-release dipyridamole alone. These results were not statistically significant.
Death Endpoint
The incidence rate of all-cause mortality was 11.3% for aspirin and extended-release dipyridamole, 11% for aspirin alone, 11.4% for extended-release dipyridamole alone and 12.3% for placebo alone. The differences between the aspirin and extended-release dipyridamole, aspirin alone and extended-release dipyridamole alone treatment groups were not statistically significant. These incidence rates for aspirin and extended-release dipyridamole and aspirin alone are consistent with previous aspirin studies in stroke and TIA patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Aspirin and extended-release Dipyridamole 25 mg/200 mg capsules are available as a hard gelatin capsule, with red opaque colored cap and ivory opaque colored body, size ‘0 Xel’ hard gelatin capsule imprinted with ‘M’ on cap and ‘25’ on body in black ink, containing yellow colored extended-release pellets of Dipyridamole and a yellow colored immediate release pellets of Aspirin.
Bottles of 60 NDC 42571-274-60
Store at 25° C (77° F); excursions permitted to 15° to 30° C (59° to 86° F) [see USP Controlled Room Temperature]. Protect from excessive moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Risk of Bleeding
Inform patients that as with other antiplatelet agents, there is a general risk of bleeding including intracranial and gastrointestinal bleeding. Inform patients about the signs and symptoms of bleeding, including occult bleeding. Tell patients to notify their physician if they are prescribed any drug which may increase risk of bleeding.
Counsel patients who consume three or more alcoholic drinks daily about the bleeding risks involved with chronic, heavy alcohol use while taking aspirin.
- Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with aspirin and extended-release dipyridamole capsules [see Use in Specific Populations (8.1)] .
- Headaches
Some patients may experience headaches upon treatment initiation; these are usually transient. In case of intolerable headaches, tell patients to contact their physician.
- Stress Test
Instruct patients who are scheduled to undergo a pharmacologic stress test to tell their healthcare provider that they are taking aspirin and extended-release dipyridamole capsules.
- Dosage and Administration
Tell patients that aspirin and extended-release dipyridamole capsules should be swallowed whole, and not chewed or crushed. If you miss a dose, continue with your next dose on your regular schedule. Do not take a double dose.
- Storage
Inform patients to protect aspirin and extended-release dipyridamole from moisture.
Manufactured by:
Micro Labs Limited
Goa-403 722, INDIA.
Manufactured for:
Micro Labs USA Inc.
Somerset, NJ 08873
Rev.10/2021
-
Patient Information
Aspirin (AS-pir-in) and extended-release dipyridamole (dye-pir-id-a-mole) Capsules
Read this Patient Information before you start taking aspirin and extended-release dipyridamole capsule and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is Aspirin and extended-release dipyridamole capsule?
Aspirin and extended-release dipyridamole capsule is a prescription medicine that contains aspirin and a medicine that is slowly released in your body, called dipyridamole. Aspirin and extended-release dipyridamole capsule is used to lower the risk of stroke in people who have had a “mini-stroke” (transient ischemic attack or TIA) or stroke due to a blood clot.
It is not known if aspirin and extended-release dipyridamole capsule is safe and effective in children. See “Who should not take aspirin and extended-release dipyridamole capsule?”
Who should not take Aspirin and extended-release dipyridamole capsule?
Do not take Aspirin and extended-release dipyridamole capsule if you:
- are allergic to any of the ingredients in aspirin and extended-release dipyridamole capsule. See the end of this leaflet for a list of ingredients in aspirin and extended-release dipyridamole capsule.
- are allergic to non-steroidal anti-inflammatory drugs (NSAIDs)
- have asthma in combination with runny nose and nasal polyps
Do not give aspirin and extended-release dipyridamole capsule to a child or teenager with a viral illness. Reye syndrome, a life-threatening condition, can happen when aspirin (an ingredient in aspirin and extended-release dipyridamole capsule) is used in children and teenagers who have certain viral illnesses.
What should I tell my doctor before using aspirin and extended-release dipyridamole capsule?
Before taking aspirin and extended-release dipyridamole capsule, tell your healthcare provider if you:
- have stomach ulcers
- have a history of bleeding problems
- have heart problems
- have kidney or liver problems
- have low blood pressure
- have myasthenia gravis
- have any other medical conditions
- are pregnant or plan to become pregnant.You should not take aspirin and extended-release dipyridamole capsule during pregnancy without first talking to your healthcare provider. Tell your healthcare provider right away if you become pregnant while taking aspirin and extended-release dipyridamole capsule.
- are breast-feeding or plan to breast-feed. Aspirin and extended-release dipyridamole capsule can pass into your milk. Talk to your healthcare provider about the best way to feed your baby if you take aspirin and extended-release dipyridamole capsule.
Tell your doctor you are taking aspirin and extended-release dipyridamole capsules if you are scheduled to have a stress test for your heart.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Aspirin and extended-release dipyridamole capsule and other medicines may affect each other causing side effects. Aspirin and extended-release dipyridamole capsule may affect the way other medicines work, and other medicines may affect how aspirin and extended-release dipyridamole capsule works.
Especially tell your healthcare provider if you take:
- a medicine for high blood pressure, irregular heart beat, or heart failure
- acetazolamide [Diamox ®]
- any blood thinner medicines
- warfarin sodium [Coumadin ®, Jantoven ®]
- a heparin medicine
- anagrelide [Agrylin ®]
- a seizure medicine
- a medicine for Alzheimer’s disease
- a water pill
- methotrexate sodium [Trexall ®]
- aspirin or a non-steroidal anti-inflammatory drug (NSAIDs). You should not take NSAIDs during treatment with aspirin and extended-release dipyridamole capsule. Using these medicines with aspirin and extended-release dipyridamole capsule can increase your risk of bleeding.
- a medicine for diabetes
- probenecid [Probalan ®, Col-Probenecid ®]
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show your healthcare provider and pharmacist when you get a new medicine.
How should I take aspirin and extended-release dipyridamole capsule?
- Take aspirin and extended-release dipyridamole capsule exactly as prescribed. Your healthcare provider will tell you how many aspirin and extended-release dipyridamole capsule to take and when to take them.
- Headaches are not uncommon when you first start taking aspirin and extended-release dipyridamole capsule, but often lessen as treatment continues. Tell your healthcare provider if you have a severe headache. Your healthcare provider may change the instructions for taking aspirin and extended-release dipyridamole capsule.
- Swallow aspirin and extended-release dipyridamole capsule whole. Do not crush or chew the capsules.
- You can take aspirin and extended-release dipyridamole capsule with or without food.
- If you miss a dose, take your next dose at the usual time. Do not take two doses at one time.
- If you take more aspirin and extended-release dipyridamole capsule (overdose) than prescribed, call your healthcare provider or Poison Control Center, or get emergency help right away.
Symptoms of an overdose of aspirin and extended-release dipyridamole capsule include:
- a warm feeling or flushing
- sweating
- restlessness
- weakness or dizziness
- a fast heart rate
- ringing in the ears
What should I avoid while using aspirin and extended-release dipyridamole capsule?
- heavy alcohol use. People who drink three or more alcoholic drinks every day have a higher risk of bleeding during treatment with aspirin and extended-release dipyridamole capsule, because it contains aspirin.
What are the possible side effects of aspirin and extended-release dipyridamole capsule?
Aspirin and extended-release dipyridamole capsule may cause serious side effects, including:
- increased risk of bleeding. You may bleed more easily during aspirin and extended-release dipyridamole capsule treatment, and it may take longer than usual for bleeding to stop. This can include:
- bleeding into your brain (intracranial hemorrhage). This can be a medical emergency. Get medical help right away if you have any of these symptoms while taking aspirin and extended-release dipyridamole capsule:
- severe headache with drowsiness
- confusion or memory change
- pass out (become unconscious)
- bleeding in your stomach or intestine.
- stomach pain
- heartburn or nausea
- vomiting blood or vomit looks like “coffee grounds”
- red or bloody stools
- black stools that look like tar
- new or worsening chest pain in some people with heart disease. Tell your healthcare provider if you have new chest pain or have any change in your chest pain during treatment with aspirin and extended-release dipyridamole capsule.
- liver problems, including increased liver function tests and liver failure. Tell your healthcare provider if you have any of these symptoms of a liver problem while taking aspirin and extended-release dipyridamole capsule:
- loss of appetite
- pale colored stool
- stomach area (abdomen) pain
- yellowing of your skin or whites of your eyes
- dark urine
- itching
Call your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects of aspirin and extended-release dipyridamole capsule include:
- headache
- upset stomach
- diarrhea
These are not all the possible side effects of aspirin and extended-release dipyridamole capsule. Tell your healthcare provider or pharmacist if you have any side effect that bothers you or that does not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store aspirin and extended-release dipyridamole capsule?
- Store aspirin and extended-release dipyridamole capsule at room temperature 59 ºF to 86 ºF (15 ºC to 30 ºC).
- Keep aspirin and extended-release dipyridamole capsules dry.
Keep aspirin and extended-release dipyridamole capsule and all medicines out of the reach of children.
General information about aspirin and extended-release dipyridamole capsule
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not use aspirin and extended-release dipyridamole capsule for a condition for which it was not prescribed. Do not give aspirin and extended-release dipyridamole capsule to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about aspirin and extended-release dipyridamole capsule. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about aspirin and extended-release dipyridamole capsule that is written for health professionals.
For more information call Micro Labs USA, Inc. at 1-855-839-8195
What are the ingredients in aspirin and extended-release dipyridamole capsule?
Active Ingredients: dipyridamole in an extended-release form and aspirin
Inactive Ingredients: caprylic/capric mono/diglycerides, D&C yellow #10 Aluminum Lake, FD&C blue #1/brilliant blue FCF Aluminum Lake, glyceryl monostearate, hypromellose, methacrylic acid and methacrylate copolymer, microcrystalline cellulose, povidone K30, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, talc, tartaric acid, titanium dioxide, triacetin and triethyl citrate.
Each capsule shell contains gelatin, ferric oxide red, ferric oxide yellow, titanium dioxide and water.
Black imprint ink composition contains ferrosoferric oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
Manufactured by:
Micro Labs Limited
Goa-403 722, INDIA.
Manufactured for:
Micro Labs USA Inc.
Somerset, NJ 08873
Rev.10/2021
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASPIRIN AND EXTENDED-RELEASE DIPYRIDAMOLE
aspirin and extended-release dipyridamole capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42571-274 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 25 mg DIPYRIDAMOLE (UNII: 64ALC7F90C) (DIPYRIDAMOLE - UNII:64ALC7F90C) DIPYRIDAMOLE 200 mg Inactive Ingredients Ingredient Name Strength CAPRYLIC/CAPRIC MONO/DIGLYCERIDES (UNII: U72Q2I8C85) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: MB5IUD6JUA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) POLYVINYL ALCOHOL (UNII: 532B59J990) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color red (red opaque colored cap and ivory opaque colored body) Score no score Shape CAPSULE (capsule) Size 24mm Flavor Imprint Code M25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42571-274-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/16/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209929 08/16/2021 Labeler - Micro Labs Limited (862174955) Establishment Name Address ID/FEI Business Operations Micro Labs Limited 915793658 analysis(42571-274) , label(42571-274) , manufacture(42571-274) , pack(42571-274)


