Label: BUSPIRONE HYDROCHLORIDE tablet
-
NDC Code(s):
65841-781-01,
65841-781-05,
65841-781-10,
65841-781-30, view more65841-781-77, 65841-782-01, 65841-782-05, 65841-782-10, 65841-782-30, 65841-782-77, 65841-783-01, 65841-783-05, 65841-783-10, 65841-783-14, 65841-783-28, 65841-783-30, 65841-783-77, 65841-784-10, 65841-784-14, 65841-784-30, 65841-784-77, 65841-842-01, 65841-842-05, 65841-842-10, 65841-842-30
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated March 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
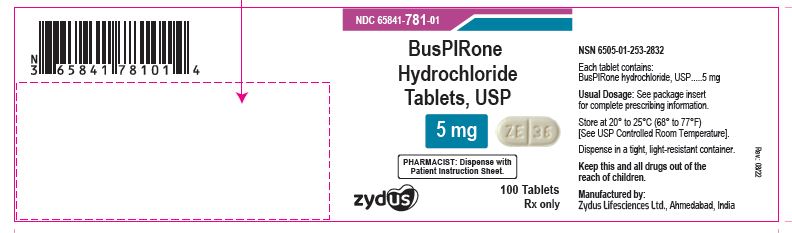
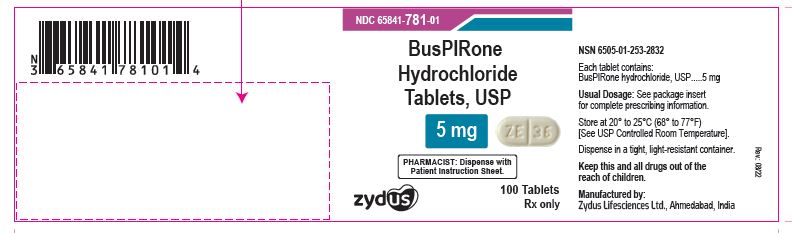
NDC 65841-781-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 5 mg
Rx only
100 tablets
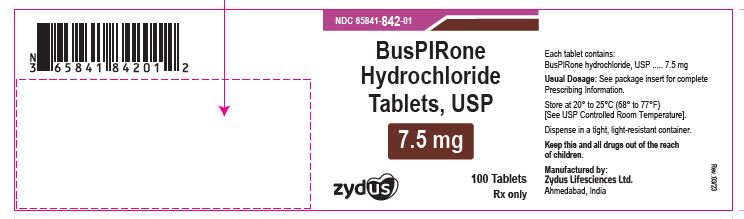
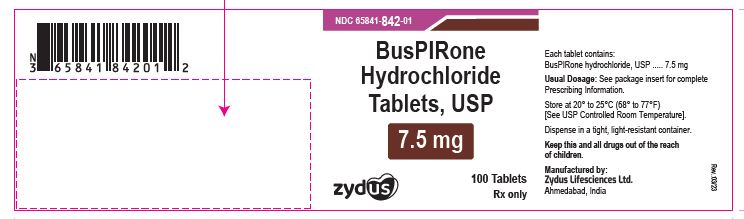
NDC 65841-842-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 7.5 mg
Rx only
100 tablets
NDC 65841-782-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 10 mg
Rx only
100 tablets

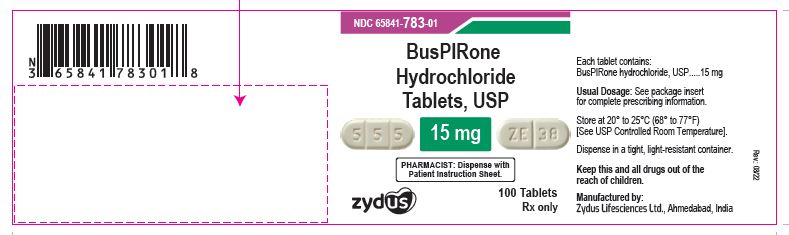
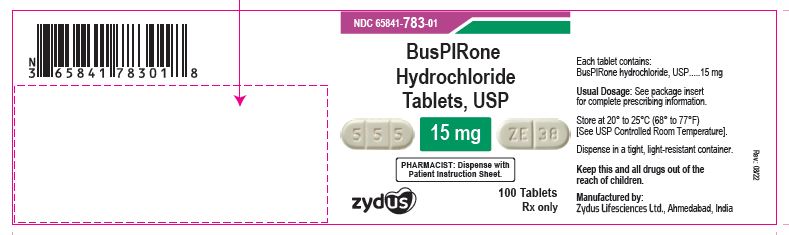
NDC 65841-783-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 15 mg
Rx only
100 tablets
NDC 65841-784-14 in bottle of 60 tablets
Buspirone Hydrochloride Tablets USP, 30 mg
Rx only
60 tablets

-
INGREDIENTS AND APPEARANCE
BUSPIRONE HYDROCHLORIDE
buspirone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-781 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUSPIRONE HYDROCHLORIDE (UNII: 207LT9J9OC) (BUSPIRONE - UNII:TK65WKS8HL) BUSPIRONE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape CAPSULE (CAPSULE) Size 8mm Flavor Imprint Code ZE;36 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-781-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 2 NDC:65841-781-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 3 NDC:65841-781-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 4 NDC:65841-781-77 100 in 1 CARTON 05/03/2014 4 NDC:65841-781-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078888 05/03/2014 BUSPIRONE HYDROCHLORIDE
buspirone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-782 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUSPIRONE HYDROCHLORIDE (UNII: 207LT9J9OC) (BUSPIRONE - UNII:TK65WKS8HL) BUSPIRONE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape CAPSULE (CAPSULE) Size 10mm Flavor Imprint Code ZE;37 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-782-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 2 NDC:65841-782-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 3 NDC:65841-782-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 4 NDC:65841-782-77 100 in 1 CARTON 05/03/2014 4 NDC:65841-782-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078888 05/03/2014 BUSPIRONE HYDROCHLORIDE
buspirone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-783 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUSPIRONE HYDROCHLORIDE (UNII: 207LT9J9OC) (BUSPIRONE - UNII:TK65WKS8HL) BUSPIRONE HYDROCHLORIDE 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (white to off-white) Score 3 pieces Shape CAPSULE (CAPSULE) Size 12mm Flavor Imprint Code 5;ZE;38 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-783-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 2 NDC:65841-783-28 180 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 3 NDC:65841-783-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 4 NDC:65841-783-77 100 in 1 CARTON 05/03/2014 4 NDC:65841-783-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:65841-783-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 6 NDC:65841-783-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078888 05/03/2014 BUSPIRONE HYDROCHLORIDE
buspirone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-784 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUSPIRONE HYDROCHLORIDE (UNII: 207LT9J9OC) (BUSPIRONE - UNII:TK65WKS8HL) BUSPIRONE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (white to off-white) Score 3 pieces Shape CAPSULE (CAPSULE) Size 17mm Flavor Imprint Code 10;ZE;39 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-784-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 2 NDC:65841-784-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2014 3 NDC:65841-784-77 100 in 1 CARTON 05/03/2014 3 NDC:65841-784-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078888 05/03/2014 BUSPIRONE HYDROCHLORIDE
buspirone hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-842 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUSPIRONE HYDROCHLORIDE (UNII: 207LT9J9OC) (BUSPIRONE - UNII:TK65WKS8HL) BUSPIRONE HYDROCHLORIDE 7.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape CAPSULE (CAPSULE) Size 9mm Flavor Imprint Code 6;23 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-842-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/21/2023 2 NDC:65841-842-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/21/2023 3 NDC:65841-842-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/21/2023 4 NDC:65841-842-30 100 in 1 CARTON 03/21/2023 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078888 03/21/2023 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(65841-781, 65841-782, 65841-783, 65841-784, 65841-842) , MANUFACTURE(65841-781, 65841-782, 65841-783, 65841-784, 65841-842)