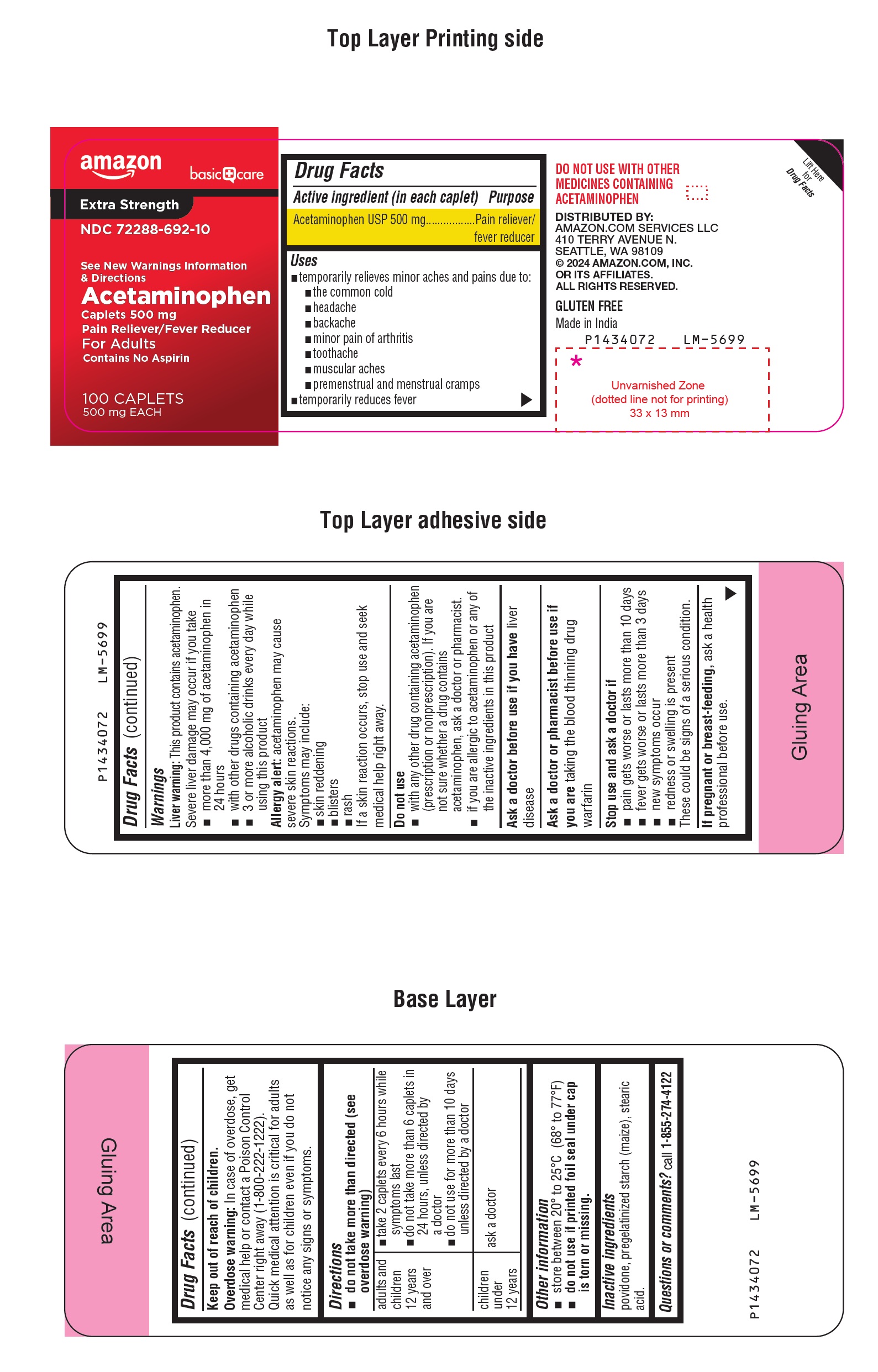
Label: AMAZON BASIC CARE ACETAMINOPHEN- acetaminophen tablet
- NDC Code(s): 72288-692-01, 72288-692-10, 72288-692-20, 72288-692-50
- Packager: Amazon.com Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions.
Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
- Do not use
- ASK DOCTOR
- Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over
- take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
children under 12 years
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 500 mg (100 Caplets Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 500 mg (100 Caplets Bottle Carton)
-
INGREDIENTS AND APPEARANCE
AMAZON BASIC CARE ACETAMINOPHEN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72288-692 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) PALMITOSTEARIC ACID (UNII: Q8Y7S3B85M) Product Characteristics Color WHITE (White to off White) Score no score Shape CAPSULE (Biconvex) Size 18mm Flavor Imprint Code N79 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72288-692-10 1 in 1 CARTON 05/15/2024 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:72288-692-20 200 in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2024 3 NDC:72288-692-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2024 4 NDC:72288-692-01 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 05/15/2024 Labeler - Amazon.com Services LLC (128990418) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650844777 ANALYSIS(72288-692) , MANUFACTURE(72288-692)