Label: DEXTROSE- dextrose monohydrate injection, solution
-
NDC Code(s):
0409-7100-02,
0409-7100-04,
0409-7100-66,
0409-7100-67, view more0409-7100-68, 0409-7100-69
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
5% Dextrose Injection, USP solution is sterile and nonpyrogenic. It is a parenteral solution containing 50 mg/mL of dextrose, hydrous in water for injection and is intended for intravenous administration after admixing with an ADD-Vantage vial, or single-dose powdered drug vials with 20 mm closure using the ADD-Vantage ADDAPTORTM (WARNING: DO NOT USE WITH CHEMOTHERAPY AGENTS).
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
The solution is slightly hypotonic (253 mOsmol/liter; calc.) in relation to the extracellular fluid (approx. 280 mOsmol/liter). The pH of the solution is 4.3 (3.2 - 6.5).
The solution is a parenteral fluid and nutrient replenisher.
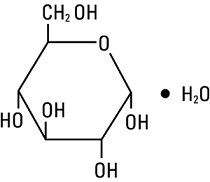
Dextrose, USP is chemically designated D-glucose, monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
CLINICAL PHARMACOLOGY
When administered intravenously, this solution provides a source of water and carbohydrate.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirements range from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
-
INDICATIONS AND USAGE
Intravenous solutions containing dextrose are indicated for parenteral replenishment of fluid and minimal carbohydrate calories as required by the clinical condition of the patient.
In this dosage form, 5% Dextrose Injection, USP is intended to be used as a diluent for the contents of an ADD-Vantage vial, or single-dose vials with 20 mm closure using the ADD-Vantage ADDAPTORTM.
- CONTRAINDICATIONS
-
WARNINGS
Excessive administration of potassium-free solutions may result in significant hypokalemia.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Studies with solutions from ADD-Vantage flexible plastic containers have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy: Animal reproduction studies have not been conducted with dextrose. It is also not known whether dextrose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose should only be given to a pregnant woman if clearly needed.
Nursing Mothers: Caution should be exercised when solutions from ADD-Vantage flexible containers are administered to a nursing woman.
Pediatric Use: The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolarity and possible intracerebral hemorrhage.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
-
DOSAGE AND ADMINISTRATION
The dose is dependent on the age, weight and clinical condition of the patient.
As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
-
HOW SUPPLIED
5% Dextrose Injection, USP is supplied in single-dose flexible plastic ADD-VantageTM diluent containers.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Unit of Sale Volume NDC 0409-7100-66
Case of 50 – 50 mL bags50 mL bags NDC 0409-7100-67
Case of 50 – 100 mL bags100 mL bags NDC 0409-7100-02
Case of 24 – 250 mL bags250 mL bags INSTRUCTIONS FOR USE WITH ADD-VANTAGE VIAL
These instructions for use should be made available to the individuals who perform the reconstitution steps.
To Open:
Peel overwrap at corner and remove solution container. Use unit within 30 days of opening overwrap, as long as the use date does not exceed the printed expiration date. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- 1.
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
- a.
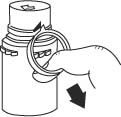
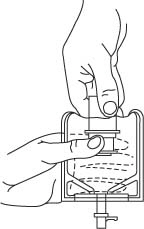
- To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (SEE FIGURE 1.), then pull straight up to remove the cap. (SEE FIGURE 2.)
NOTE: Once the breakaway cap has been removed, do not access vial with syringe.
-
- b.
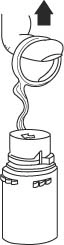
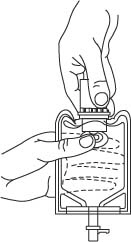
- To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (SEE FIGURE 3.)
- 2.
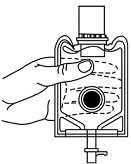
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (SEE FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.
NOTE: Once vial is seated, do not attempt to remove. (SEE FIGURE 4.) - 3.
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- 4.
- Label appropriately.
Fig. 3 Fig. 4
Preparation for Administration:
(Use Aseptic Technique)
- 1.
- Confirm the activation and admixture of vial contents.
- 2.
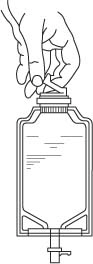
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- 3.
- Close flow control clamp of administration set.
- 4.
- Remove cover from outlet port at bottom of container.
- 5.
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated. NOTE: See full directions on administration set carton.
- 6.
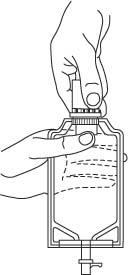
- Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- 7.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- 8.
- Open flow control clamp and clear air from set. Close clamp.
- 9.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- 10.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
INSTRUCTIONS FOR USE WITH ADD-VANTAGE ADDAPTORTM
The instructions for use provided with the ADD-Vantage ADDAPTORTM should be made available to the individuals who perform the reconstitution steps.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA

LAB-1143-3.0
Revised: 09/2021
-
PRINCIPAL DISPLAY PANEL - 50 mL Bag Label
PULL INNER PLUG/STOPPER
AND MIX DRUG BEFORE USEADD-Vantage™ Unit
NDC 0409-7100-685% DEXTROSE
Injection, USP50
mLEACH 100 mL CONTAINS DEXTROSE, HYDROUS 5 g.
253 mOsmol/LITER (calc.). pH 4.3 (3.2 TO 6.5)
DEXTROSE SOLUTIONS WITHOUT SALTS SHOULD
NOT BE USED IN BLOOD TRANSFUSIONS BECAUSE
OF POSSIBLE ROULEAU FORMATION. FOR USE ONLY
WITH ADD-Vantage™ SYSTEM COMPONENTS. SINGLE-
DOSE CONTAINER. FOR IV USE. USUAL DOSAGE: SEE
PACKAGE INSERT. STERILE, NONPYROGENIC. USE ONLY
IF SOLUTION IS CLEAR AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES CONNECTIONS.DISTRIBUTED BY
HOSPIRA, INC.,
LAKE FOREST, IL 60045 USARx ONLY
IM-4480
144752013
v
CONTAINS DEHP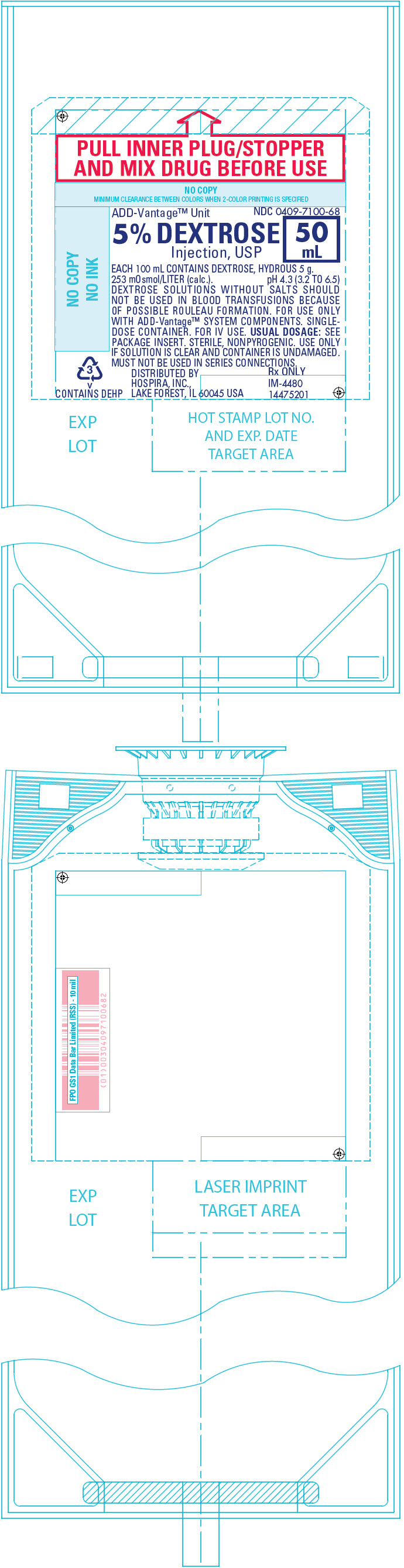
-
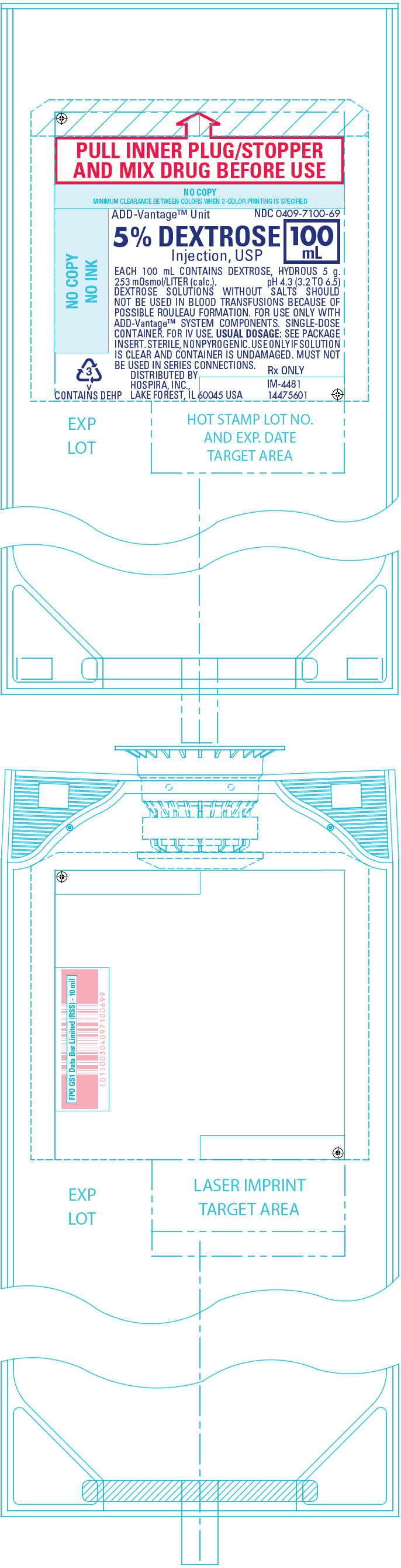
PRINCIPAL DISPLAY PANEL - 100 mL Bag Label
PULL INNER PLUG/STOPPER
AND MIX DRUG BEFORE USEADD-Vantage™ Unit
NDC 0409-7100-695% DEXTROSE
Injection, USP100
mLEACH 100 mL CONTAINS DEXTROSE, HYDROUS 5 g.
253 mOsmol/LITER (calc.). pH 4.3 (3.2 TO 6.5)
DEXTROSE SOLUTIONS WITHOUT SALTS SHOULD
NOT BE USED IN BLOOD TRANSFUSIONS BECAUSE OF
POSSIBLE ROULEAU FORMATION. FOR USE ONLY WITH
ADD-Vantage™ SYSTEM COMPONENTS. SINGLE-DOSE
CONTAINER. FOR IV USE. USUAL DOSAGE: SEE PACKAGE
INSERT. STERILE, NONPYROGENIC. USE ONLY IF SOLUTION
IS CLEAR AND CONTAINER IS UNDAMAGED. MUST NOT
BE USED IN SERIES CONNECTIONS.DISTRIBUTED BY
HOSPIRA, INC.,
LAKE FOREST, IL 60045 USARx ONLY
IM-4481
144756013
v
CONTAINS DEHP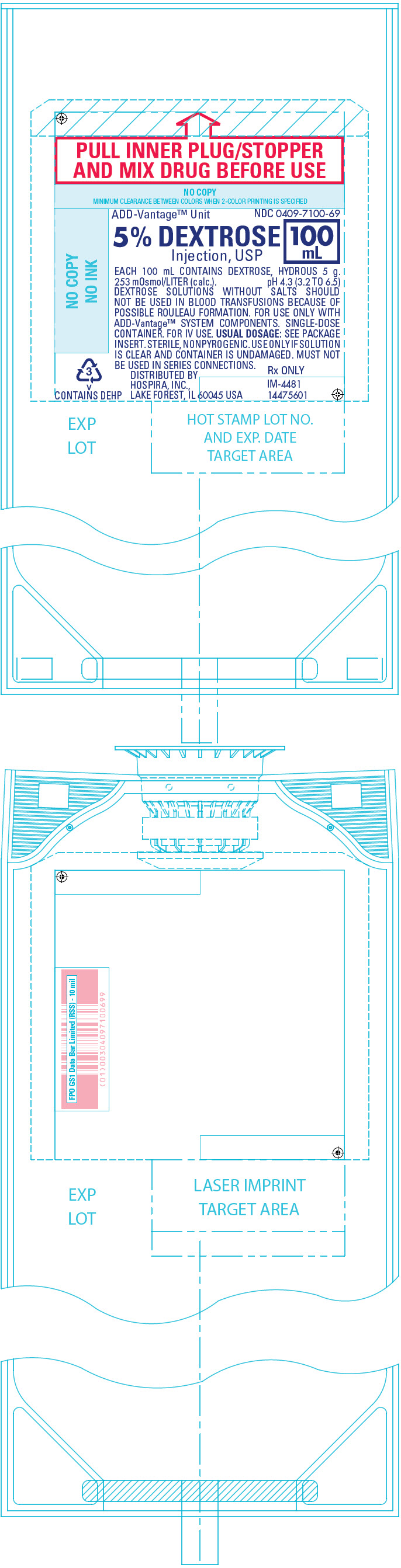
-
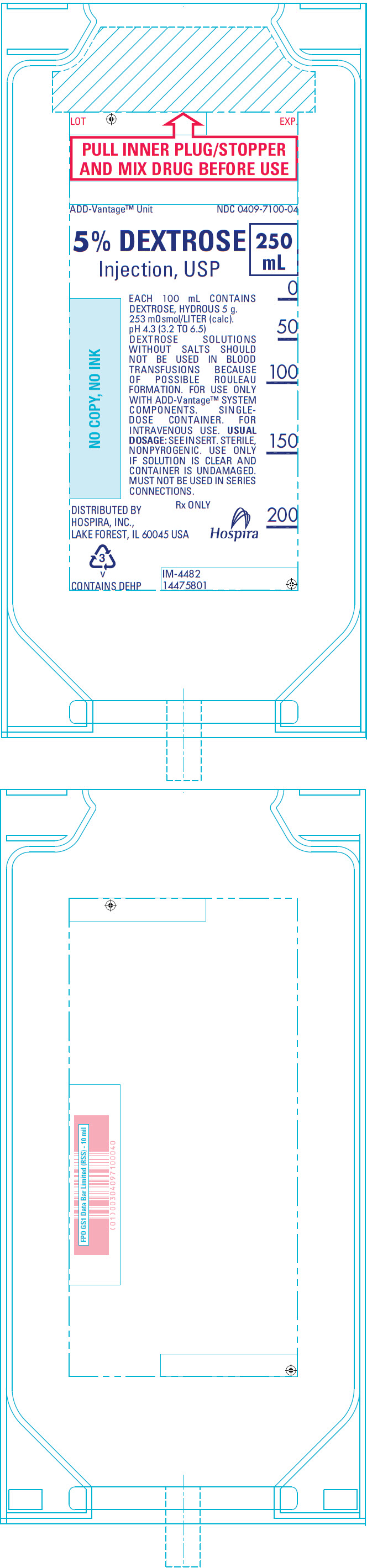
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label
LOT
EXP.PULL INNER PLUG/STOPPER
AND MIX DRUG BEFORE USEADD-Vantage™ Unit
NDC 0409-7100-045% DEXTROSE
Injection, USP250
mLEACH 100 mL CONTAINS
DEXTROSE, HYDROUS 5 g.
253 mOsmol/LITER (calc).
pH 4.3 (3.2 TO 6.5)
DEXTROSE SOLUTIONS
WITHOUT SALTS SHOULD
NOT BE USED IN BLOOD
TRANSFUSIONS BECAUSE
OF POSSIBLE ROULEAU
FORMATION. FOR USE ONLY
WITH ADD-Vantage™ SYSTEM
COMPONENTS. SINGLE-
DOSE CONTAINER. FOR
INTRAVENOUS USE. USUAL
DOSAGE: SEE INSERT. STERILE,
NONPYROGENIC. USE ONLY
IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES
CONNECTIONS.Rx ONLY
DISTRIBUTED BY
HOSPIRA, INC.,
LAKE FOREST, IL 60045 USAHospira
3
v
CONTAINS DEHPIM-4482
14475801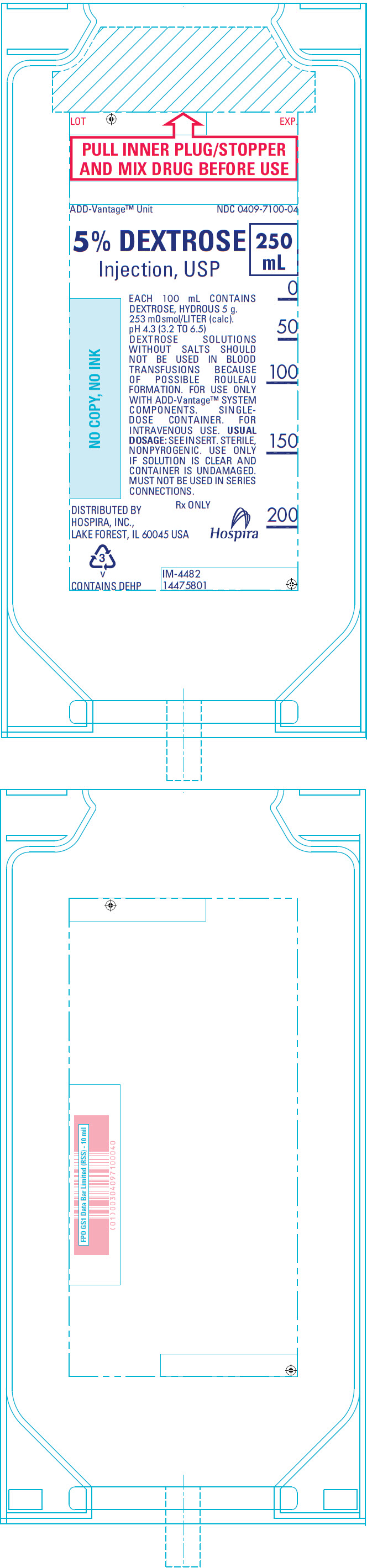
-
PRINCIPAL DISPLAY PANEL - OVERWRAP – WR-0583
One/ADD-Vantage™ Unit
TO OPEN–PEEL AT CORNER
For use only with ADD-Vantage™ system components.
The overwrap is a moisture barrier. Use unit within 30 days of opening
overwrap, as long as the use date does not exceed the printed
expiration date. Store at 20 to 25°C (68 to 77°F). [See USP Controlled
Room Temperature.] Protect from freezing. See insert.
After removing the overwrap, check for minute leaks by
squeezing container firmly. If leaks are found, discard
unit as sterility may be impaired.Rx only
14475901Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Hospira
WR-0583

-
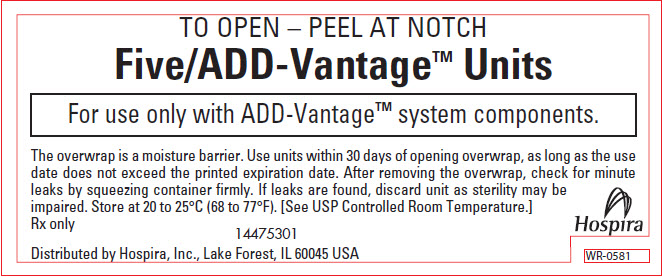
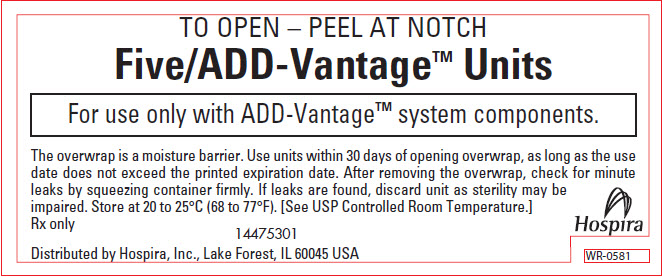
PRINCIPAL DISPLAY PANEL - OVERWRAP – WR-0581
TO OPEN – PEEL AT NOTCH
Five/ADD-Vantage™ Units
For use only with ADD-Vantage™ system components.
The overwrap is a moisture barrier. Use units within 30 days of opening overwrap, as long as the use
date does not exceed the printed expiration date. After removing the overwrap, check for minute
leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be
impaired. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]Rx only
14475301Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Hospira
WR-0581
-
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-7100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-7100-66 10 in 1 CASE 08/04/2005 1 5 in 1 POUCH 1 NDC:0409-7100-68 50 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:0409-7100-67 10 in 1 CASE 09/14/2005 2 5 in 1 POUCH 2 NDC:0409-7100-69 100 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:0409-7100-02 12 in 1 CASE 06/27/2005 3 2 in 1 POUCH 3 NDC:0409-7100-04 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019466 06/27/2005 Labeler - Hospira, Inc. (141588017)