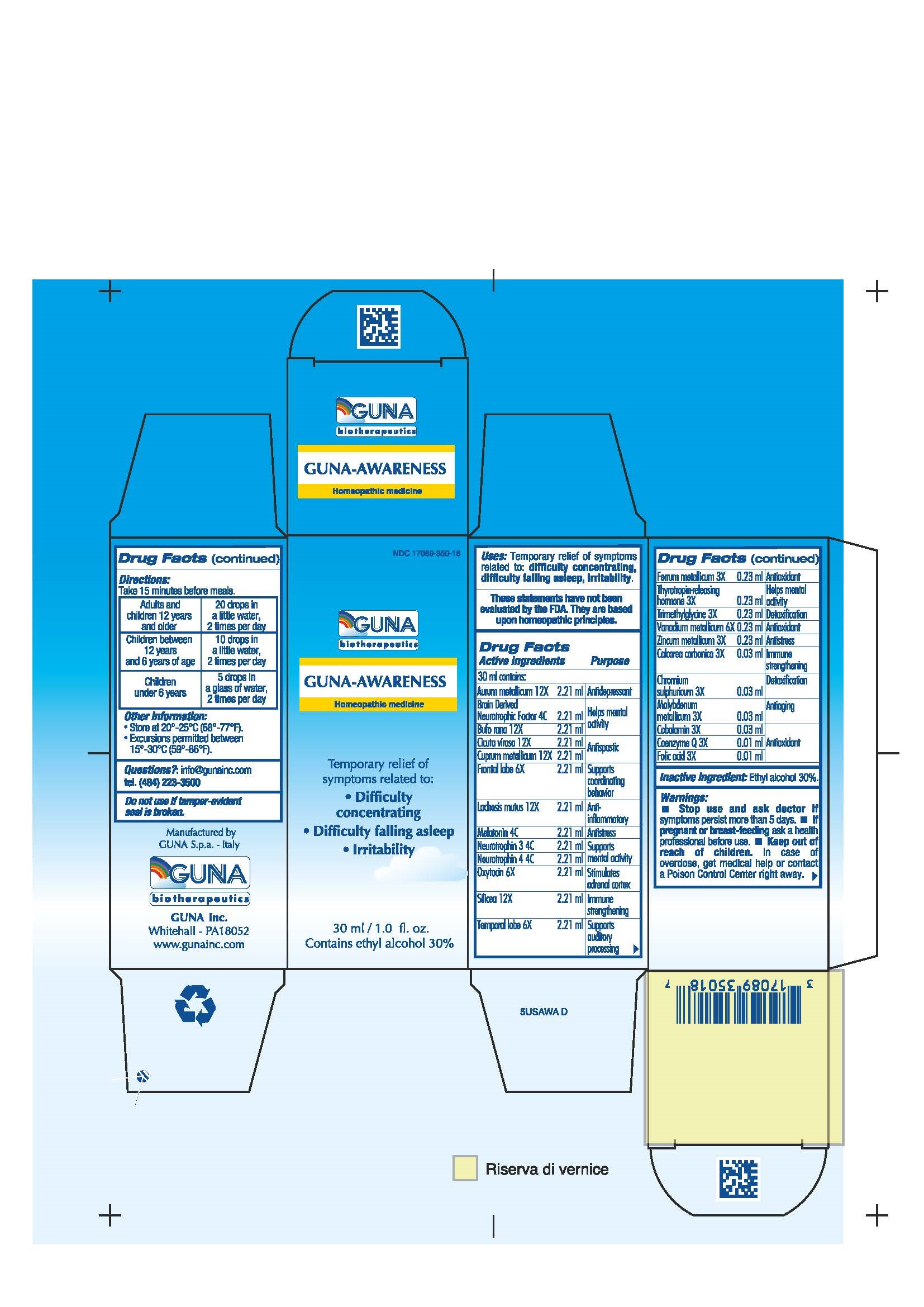
Label: GUNA-AWARENESS- betaine - bufo bufo cutaneous gland - calcium carbonate - chromic sulfate - cicuta virosa root - copper - folic acid - gold - cobalamin - iron - lachesis muta venom - melatonin - molybdenum - neurotrophin-3 - neurotrophin-4 - oxytocin - silicon dioxide - sus scrofa frontal lobe - sus scrofa temporal lobe - thyrotropin alfa - ubidecarenone - vanadium - zinc - brain-derived neurotrophic factor human - solution/ drops
- NDC Code(s): 17089-350-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS/PURPOSE
AURUM METALLICUM 12X ANTIDEPRESSANT
BRAIN DERIVED NEUROTROPHIC FACTOR 4C HELPS MENTAL ACTIVITY
BUFO RANA 12X HELPS MENTAL ACTIVITY
CALCAREA CARBONICA 3X IMMUNE STRENGTHENING
CHROMIUM SULPHURICUM 3X DETOXIFICATION
CICUTA VIROSA 12X ANTISPASTIC
COBALAMIN 3X ANTIOXIDANT
COENZYME Q 3X ANTIOXIDANT
CUPRUM METALLICUM 12X ANTISPASTIC
FERRUM METALLICUM 3X ANTIOXIDANT
FOLIC ACID 3X ANTIOXIDANT
FRONTAL LOBE 6X SUPPORTS COORDINATING BEHAVIOR
LACHESIS MUTUS 12X ANTI-INFLAMMATORY
MELATONIN 4C ANTISTRESS
MOLYBDENUM METALLICUM 3X ANTIAGING
NEUROTROPHIN 3 4C SUPPORTS MENTAL ACTIVITY
NEUROTROPHIN 4 4C SUPPORTS MENTAL ACTIVITY
OXYTOCIN 6X STIMULATES ADRENAL CORTEX
SILICEA 12X IMMUNE STRENGTHENING
TEMPORAL LOBE 6X SUPPORTS AUDITORY PROCESSING
THYROTROPIN-RELEASING HORMONE 3X HELPS MENTAL ACTIVITY
TRIMETHYLGLYCINE 3X DETOXIFICATION
VANADIUM METALLICUM 6X ANTIOXIDANT
ZINCUM METALLICUM 3X ANTISTRESS
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-AWARENESS
betaine - bufo bufo cutaneous gland - calcium carbonate - chromic sulfate - cicuta virosa root - copper - folic acid - gold - cobalamin - iron - lachesis muta venom - melatonin - molybdenum - neurotrophin-3 - neurotrophin-4 - oxytocin - silicon dioxide - sus scrofa frontal lobe - sus scrofa temporal lobe - thyrotropin alfa - ubidecarenone - vanadium - zinc - brain-derived neurotrophic factor human - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-350 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 30 mL BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN (UNII: 7171WSG8A2) (BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN - UNII:7171WSG8A2) BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN 4 [hp_C] in 30 mL BUFO BUFO CUTANEOUS GLAND (UNII: Q59QU6N72Q) (BUFO BUFO CUTANEOUS GLAND - UNII:Q59QU6N72Q) BUFO BUFO CUTANEOUS GLAND 12 [hp_X] in 30 mL CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 3 [hp_X] in 30 mL CHROMIC SULFATE (UNII: Y0C99N5TMZ) (CHROMIC CATION - UNII:X1N4508KF1) CHROMIC SULFATE 3 [hp_X] in 30 mL CICUTA VIROSA ROOT (UNII: YEA9P21S8N) (CICUTA VIROSA ROOT - UNII:YEA9P21S8N) CICUTA VIROSA ROOT 12 [hp_X] in 30 mL COBALAMIN (UNII: 8406EY2OQA) (COBALAMIN - UNII:8406EY2OQA) COBALAMIN 3 [hp_X] in 30 mL UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 3 [hp_X] in 30 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] in 30 mL IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 3 [hp_X] in 30 mL FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 3 [hp_X] in 30 mL SUS SCROFA FRONTAL LOBE (UNII: GV54Q19G55) (SUS SCROFA FRONTAL LOBE - UNII:GV54Q19G55) SUS SCROFA FRONTAL LOBE 6 [hp_X] in 30 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 12 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL MOLYBDENUM (UNII: 81AH48963U) (MOLYBDENUM - UNII:81AH48963U) MOLYBDENUM 3 [hp_X] in 30 mL NEUROTROPHIN-3 (UNII: 02R4V6T25Y) (NEUROTROPHIN-3 - UNII:02R4V6T25Y) NEUROTROPHIN-3 4 [hp_C] in 30 mL NEUROTROPHIN-4 (UNII: P658DCA9XD) (NEUROTROPHIN-4 - UNII:P658DCA9XD) NEUROTROPHIN-4 4 [hp_C] in 30 mL OXYTOCIN (UNII: 1JQS135EYN) (OXYTOCIN - UNII:1JQS135EYN) OXYTOCIN 6 [hp_X] in 30 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 30 mL SUS SCROFA TEMPORAL LOBE (UNII: 490XI1KB4S) (SUS SCROFA TEMPORAL LOBE - UNII:490XI1KB4S) SUS SCROFA TEMPORAL LOBE 6 [hp_X] in 30 mL THYROTROPIN ALFA (UNII: AVX3D5A4LM) (THYROTROPIN ALFA - UNII:AVX3D5A4LM) THYROTROPIN ALFA 3 [hp_X] in 30 mL BETAINE (UNII: 3SCV180C9W) (BETAINE - UNII:3SCV180C9W) BETAINE 3 [hp_X] in 30 mL VANADIUM (UNII: 00J9J9XKDE) (VANADIUM - UNII:00J9J9XKDE) VANADIUM 6 [hp_X] in 30 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 3 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-350-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/16/2008 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-350)