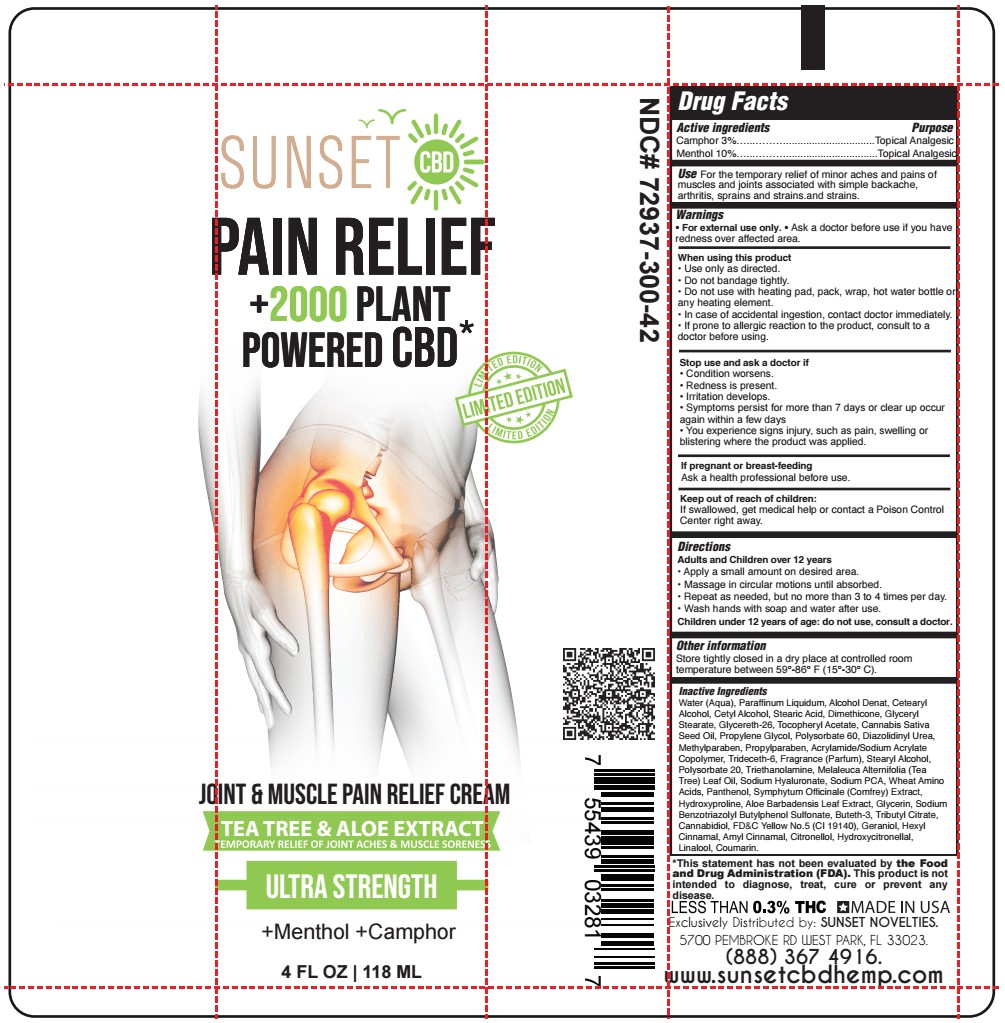
Label: MENTHOL, CAMPHOR cream
- NDC Code(s): 72937-300-42
- Packager: SUNSET NOVELTIES, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- DIRECTIONS
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Water (Aqua), Paraffinum Liquidum, Alcohol Denat, Cetearyl Alcohol, Cetyl Alcohol, Stearic Acid, Dimethicone, Glyceryl Stearate, Glycereth-26, Tocopheryl Acetate, Cannabis Sativa Seed Oil, Propylene Glycol, Polysorbate 60, Diazolidinyl Urea, Methylparaben, Propylparaben, Acrylamide/Sodium Acrylate Copolymer, Trideceth-6, Fragrance (Parfum), Stearyl Alcohol, Polysorbate 20, Triethanolamine, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Sodium Hyaluronate, Sodium PCA, Wheat Amino Acids, Panthenol, Symphytum Officinale (Comfrey) Extract, Hydroxyproline, Aloe Barbadensis Leaf Extract, Glycerin, Sodium Benzotriazolyl Butylphenol Sulfonate, Buteth-3, Tributyl Citrate, Cannabidiol, FD&C Yellow No.5 (CI 19140), Geraniol, Hexyl Cinnamal, Amyl Cinnamal, Citronellol, Hydroxycitronellal, Linalool, Coumarin.
- SUNSET - JOINT AND MUSCLE PAIN RELIEF CREAM 4 oz TUBE LIMITED EDITION
-
INGREDIENTS AND APPEARANCE
MENTHOL, CAMPHOR
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72937-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 10 g in 100 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 3 g in 100 g Inactive Ingredients Ingredient Name Strength CANNABIDIOL (UNII: 19GBJ60SN5) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PROPYLPARABEN (UNII: Z8IX2SC1OH) TROLAMINE (UNII: 9O3K93S3TK) LINALOOL, (+/-)- (UNII: D81QY6I88E) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) GLYCERETH-26 (UNII: NNE56F2N14) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) TRIDECETH-6 (UNII: 3T5PCR2H0C) TEA TREE OIL (UNII: VIF565UC2G) GERANIOL (UNII: L837108USY) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) COUMARIN (UNII: A4VZ22K1WT) COMFREY LEAF (UNII: DG4F8T839X) BUTETH-3 (UNII: OC116GRO69) AMINO ACIDS, WHEAT (UNII: 0370GZL32F) CETYL ALCOHOL (UNII: 936JST6JCN) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) HYDROXYPROLINE (UNII: RMB44WO89X) GLYCERIN (UNII: PDC6A3C0OX) TRIBUTYL CITRATE (UNII: 827D5B1B6S) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIMETHICONE 1000 (UNII: MCU2324216) POLYSORBATE 60 (UNII: CAL22UVI4M) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLYSORBATE 20 (UNII: 7T1F30V5YH) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALCOHOL (UNII: 3K9958V90M) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) SODIUM BENZOTRIAZOLYL BUTYLPHENOL SULFONATE (UNII: 0LA2QC9O3Z) PANTHENOL (UNII: WV9CM0O67Z) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72937-300-42 113 g in 1 TUBE; Type 0: Not a Combination Product 02/07/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/04/2020 Labeler - SUNSET NOVELTIES, INC (067218145)