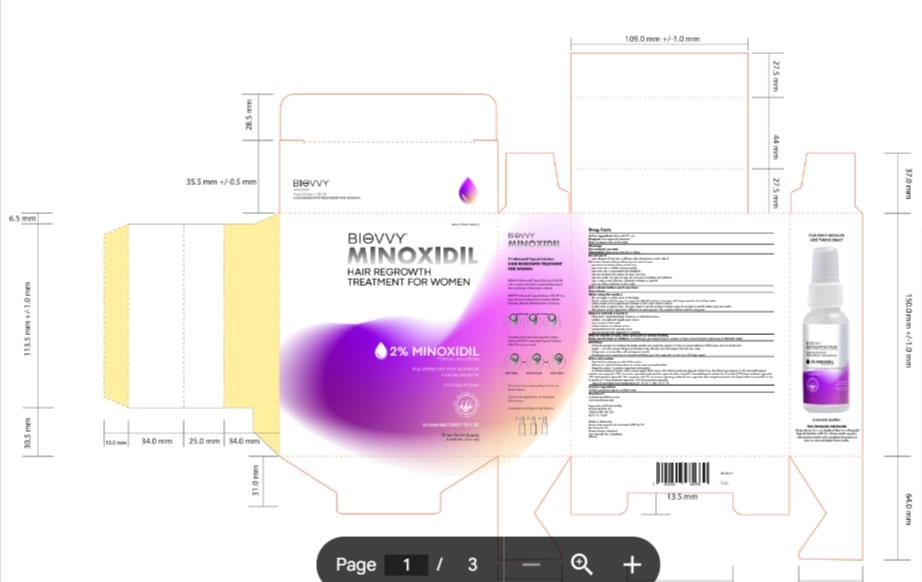
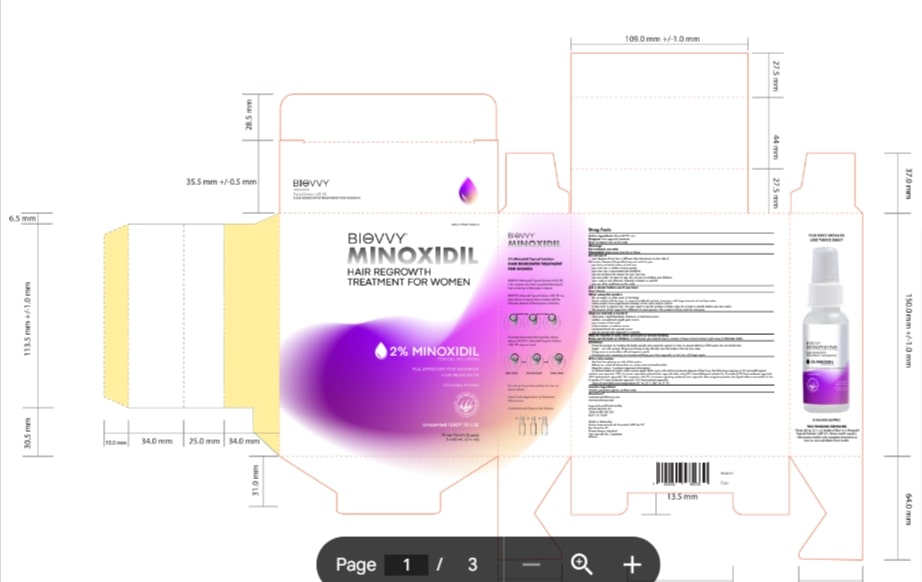
Label: MINOXIDIL 2%- minoxidil lotion
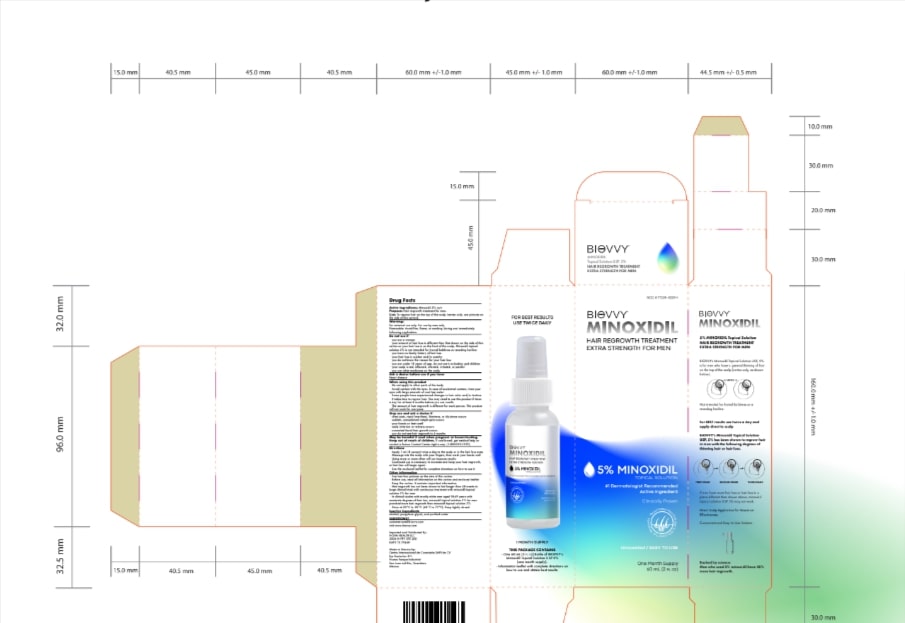
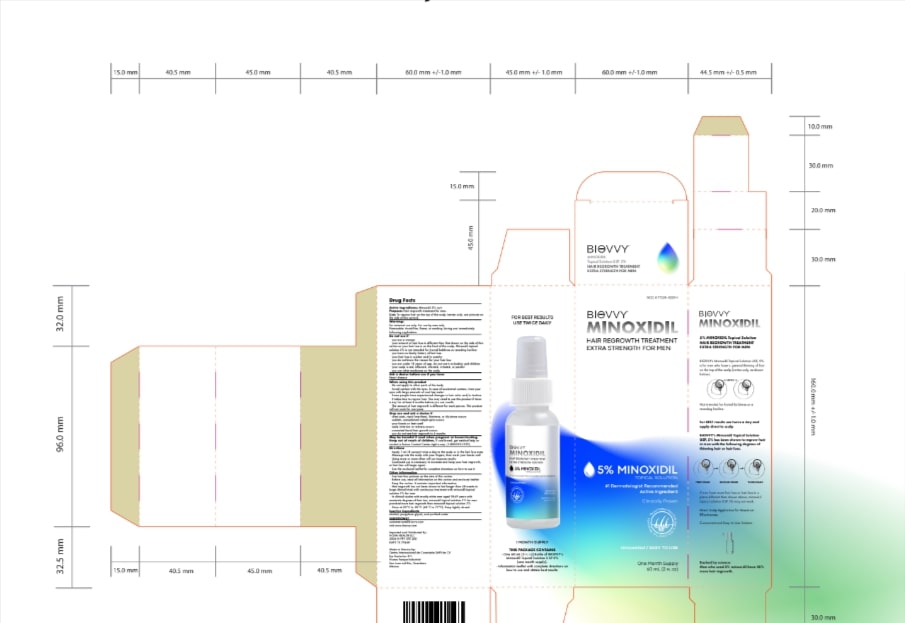
MINOXIDIL 5%- minoxidil lotion
-
NDC Code(s):
77229-050-01,
77229-050-13,
77229-050-16,
77229-099-01, view more77229-099-13, 77229-099-16
- Packager: Centro Internacional de Cosmiatría, S.A.P.I. de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNINGS
Warnings
For external use only
Flammable: Keep away from fire or flame.
Do not use if
your degree of hair loss is different than that shown on the side of this carton, because this product may not work for you
you have no family history of hair loss
your hair loss is sudden and/or patchy
your hair loss is associated with childbirth
you do not know the reason for your hair loss
you are under 18 years of age. Do not use it on babies and children.
your scalp is red, inflamed, infected, irritated, or painful
you use other medicines on the scalpAsk a doctor before use if you have
Heart disease
When using this product
Do not apply on other parts of the body.
Avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
Some people have experienced changes in hair color and/or texture.
It takes time to regrow hair. You may need to use this product 2 times a day for at least 4 months before you see results.
The amount of hair regrowth is different for each person. This product will not work for everyone.Stop use and ask a doctor if
chest pain, rapid heartbeat, faintness, or dizziness occurs
sudden, unexplained weight gain occurs
your hands or feet swell
scalp irritation or redness occurs
unwanted facial hair growth occurs
you do not see hair regrowth in 4 monthsMay be harmful if used when pregnant or breast-feeding.
-
DOSAGE & ADMINISTRATION
DIRECTIONS
Before each application, prime the sprayer by holding the bottle upright and pump the sprayer 5 times to ensure delivery of full spray. Do not inhale mist.
Apply 1 mL with sprayer (8 sprays) 2 times a day directly onto the scalp in the hair loss area.
Using more or more often will not improve results.
Continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again. - INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINOXIDIL 2%
minoxidil lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77229-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 0.02 g in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 0.25 g in 1 g ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.0093 g in 1 g BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) 0.001 g in 1 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.637 g in 1 g WATER (UNII: 059QF0KO0R) 0.0827 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77229-050-01 1 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/08/2024 2 NDC:77229-050-13 3 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/08/2024 3 NDC:77229-050-16 6 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/08/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/07/2024 MINOXIDIL 5%
minoxidil lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77229-099 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 0.02 g in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 0.25 g in 1 g ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.0093 g in 1 g BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) 0.001 g in 1 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.637 g in 1 g WATER (UNII: 059QF0KO0R) 0.0827 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77229-099-01 1 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/08/2024 2 NDC:77229-099-13 3 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/08/2024 3 NDC:77229-099-16 6 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 02/08/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/07/2024 Labeler - Centro Internacional de Cosmiatría, S.A.P.I. de C.V. (814124954) Establishment Name Address ID/FEI Business Operations Centro Internacional de Cosmiatría, S.A.P.I. de C.V. 814124954 manufacture(77229-050, 77229-099)