Label: CARBAMAZEPINE tablet
- NDC Code(s): 0615-8545-39
- Packager: NCS HealthCare of KY, LLC dba Vangard Labs
- This is a repackaged label.
- Source NDC Code(s): 51672-4005
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - Prescribing Information
-
BOXED WARNING
(What is this?)SERIOUS DERMATOLOGIC REACTIONS AND HLA-B*1502 ALLELE - SERIOUS AND SOMETIMES FATAL DERMATOLOGIC REACTIONS, INCLUDING TOXIC EPIDERMAL NECROLYSIS (TEN) AND STEVENS-JOHNSON SYNDROME (SJS), HAVE BEEN ...
WARNINGS
SERIOUS DERMATOLOGIC REACTIONS AND HLA-B*1502 ALLELE
SERIOUS AND SOMETIMES FATAL DERMATOLOGIC REACTIONS, INCLUDING TOXIC EPIDERMAL NECROLYSIS (TEN) AND STEVENS-JOHNSON SYNDROME (SJS), HAVE BEEN REPORTED DURING TREATMENT WITH CARBAMAZEPINE. THESE REACTIONS ARE ESTIMATED TO OCCUR IN 1 TO 6 PER 10,000 NEW USERS IN COUNTRIES WITH MAINLY CAUCASIAN POPULATIONS, BUT THE RISK IN SOME ASIAN COUNTRIES IS ESTIMATED TO BE ABOUT 10 TIMES HIGHER. STUDIES IN PATIENTS OF CHINESE ANCESTRY HAVE FOUND A STRONG ASSOCIATION BETWEEN THE RISK OF DEVELOPING SJS/TEN AND THE PRESENCE OF HLA-B*1502, AN INHERITED ALLELIC VARIANT OF THE HLA-B GENE. HLA-B*1502 IS FOUND ALMOST EXCLUSIVELY IN PATIENTS WITH ANCESTRY ACROSS BROAD AREAS OF ASIA. PATIENTS WITH ANCESTRY IN GENETICALLY AT-RISK POPULATIONS SHOULD BE SCREENED FOR THE PRESENCE OF HLA-B*1502 PRIOR TO INITIATING TREATMENT WITH CARBAMAZEPINE. PATIENTS TESTING POSITIVE FOR THE ALLELE SHOULD NOT BE TREATED WITH CARBAMAZEPINE UNLESS THE BENEFIT CLEARLY OUTWEIGHS THE RISK (SEE WARNINGS AND PRECAUTIONS, LABORATORY TESTS).
CloseAPLASTIC ANEMIA AND AGRANULOCYTOSIS
APLASTIC ANEMIA AND AGRANULOCYTOSIS HAVE BEEN REPORTED IN ASSOCIATION WITH THE USE OF CARBAMAZEPINE. DATA FROM A POPULATION-BASED CASE CONTROL STUDY DEMONSTRATE THAT THE RISK OF DEVELOPING THESE REACTIONS IS 5 TO 8 TIMES GREATER THAN IN THE GENERAL POPULATION. HOWEVER, THE OVERALL RISK OF THESE REACTIONS IN THE UNTREATED GENERAL POPULATION IS LOW, APPROXIMATELY SIX PATIENTS PER ONE MILLION POPULATION PER YEAR FOR AGRANULOCYTOSIS AND TWO PATIENTS PER ONE MILLION POPULATION PER YEAR FOR APLASTIC ANEMIA.
ALTHOUGH REPORTS OF TRANSIENT OR PERSISTENT DECREASED PLATELET OR WHITE BLOOD CELL COUNTS ARE NOT UNCOMMON IN ASSOCIATION WITH THE USE OF CARBAMAZEPINE, DATA ARE NOT AVAILABLE TO ESTIMATE ACCURATELY THEIR INCIDENCE OR OUTCOME. HOWEVER, THE VAST MAJORITY OF THE CASES OF LEUKOPENIA HAVE NOT PROGRESSED TO THE MORE SERIOUS CONDITIONS OF APLASTIC ANEMIA OR AGRANULOCYTOSIS.
BECAUSE OF THE VERY LOW INCIDENCE OF AGRANULOCYTOSIS AND APLASTIC ANEMIA, THE VAST MAJORITY OF MINOR HEMATOLOGIC CHANGES OBSERVED IN MONITORING OF PATIENTS ON CARBAMAZEPINE ARE UNLIKELY TO SIGNAL THE OCCURRENCE OF EITHER ABNORMALITY. NONETHELESS, COMPLETE PRETREATMENT HEMATOLOGICAL TESTING SHOULD BE OBTAINED AS A BASELINE. IF A PATIENT IN THE COURSE OF TREATMENT EXHIBITS LOW OR DECREASED WHITE BLOOD CELL OR PLATELET COUNTS, THE PATIENT SHOULD BE MONITORED CLOSELY. DISCONTINUATION OF THE DRUG SHOULD BE CONSIDERED IF ANY EVIDENCE OF SIGNIFICANT BONE MARROW DEPRESSION DEVELOPS.
-
SPL UNCLASSIFIED SECTIONBefore prescribing carbamazepine, the physician should be thoroughly familiar with the details of this prescribing information, particularly regarding use with other drugs, especially those which ...
-
DESCRIPTIONCarbamazepine USP, is an anticonvulsant and specific analgesic for trigeminal neuralgia, available for oral administration as chewable tablets of 100 and 200 mg, tablets of 200 mg ...
-
CLINICAL PHARMACOLOGYIn controlled clinical trials, carbamazepine has been shown to be effective in the treatment of psychomotor and grand mal seizures, as well as trigeminal neuralgia. Mechanism of ...
-
INDICATIONS AND USAGEEpilepsy - Carbamazepine is indicated for use as an anticonvulsant drug. Evidence supporting efficacy of carbamazepine as an anticonvulsant was derived from active drug-controlled studies that ...
-
CONTRAINDICATIONSCarbamazepine should not be used in patients with a history of previous bone marrow depression, hypersensitivity to the drug, or known sensitivity to any of the tricyclic compounds, such as ...
-
WARNINGSSerious Dermatologic Reactions - Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), have been reported with ...
-
PRECAUTIONSGeneral - Before initiating therapy, a detailed history and physical examination should be made. Carbamazepine should be used with caution in patients with a mixed seizure disorder that includes ...
-
ADVERSE REACTIONSIf adverse reactions are of such severity that the drug must be discontinued, the physician must be aware that abrupt discontinuation of any anticonvulsant drug in a responsive epileptic patient ...
-
DRUG ABUSE AND DEPENDENCENo evidence of abuse potential has been associated with carbamazepine, nor is there evidence of psychological or physical dependence in humans.
-
OVERDOSAGEAcute Toxicity - Lowest known lethal dose: adults, 3.2 g (a 24-year-old woman died of a cardiac arrest and a 24-year-old man died of pneumonia and hypoxic encephalopathy); children, 4 g (a ...
-
DOSAGE AND ADMINISTRATION(SEE TABLE BELOW) Carbamazepine suspension in combination with liquid chlorpromazine or thioridazine results in precipitate formation, and, in the case of chlorpromazine, there has been a report ...
-
DOSAGE FORMS & STRENGTHSDosage Information - Initial Dose Subsequent Dose Maximum Daily Dose - Indication Tablet* XR† Suspension Tablet* XR* Suspension Tablet* XR* Suspension - * Tablet = Chewable or ...
-
HOW SUPPLIEDCarbamazepine Tablets USP, (Chewable), 100 mg: White, flat, round tablet with pink specks, and cherry fragrance. One side scored and engraved with "TARO" above the score and "16" under the score ...
-
HOW SUPPLIEDCarbamazepine Tablets USP, 200 mg: White, round, flat beveled-edge, one side scored and engraved "TARO" above and "11" below the score, the other side plain. Blistercards of 30NDC ...
-
HOW SUPPLIEDCarbamazepine Extended-Release Tablets USP, 100 mg: White to off-white, round bi-convexed tablets engraved with "T91" on one side and plain on the other side. Carbamazepine Extended-Release ...
-
HOW SUPPLIEDCarbamazepine Oral Suspension USP, 100 mg/5 mL (teaspoonful): Orange colored and orange flavored suspension. Shake well before using. Store Carbamazepine Oral Suspension USP at 20° to 25°C ...
-
SPL UNCLASSIFIED SECTIONTrademarks are the property of their respective owners. Manufactured by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761 - Distributed by: Taro Pharmaceuticals U.S.A., Inc. ...
-
MEDICATION GUIDECarbamazepine (kar" ba maz' e peen) Tablets, Carbamazepine (kar" ba maz' e peen) Oral Suspension, Carbamazepine (kar" ba maz' e peen) Tablets (Chewable), and Carbamazepine (kar" ba maz' e peen ...
-
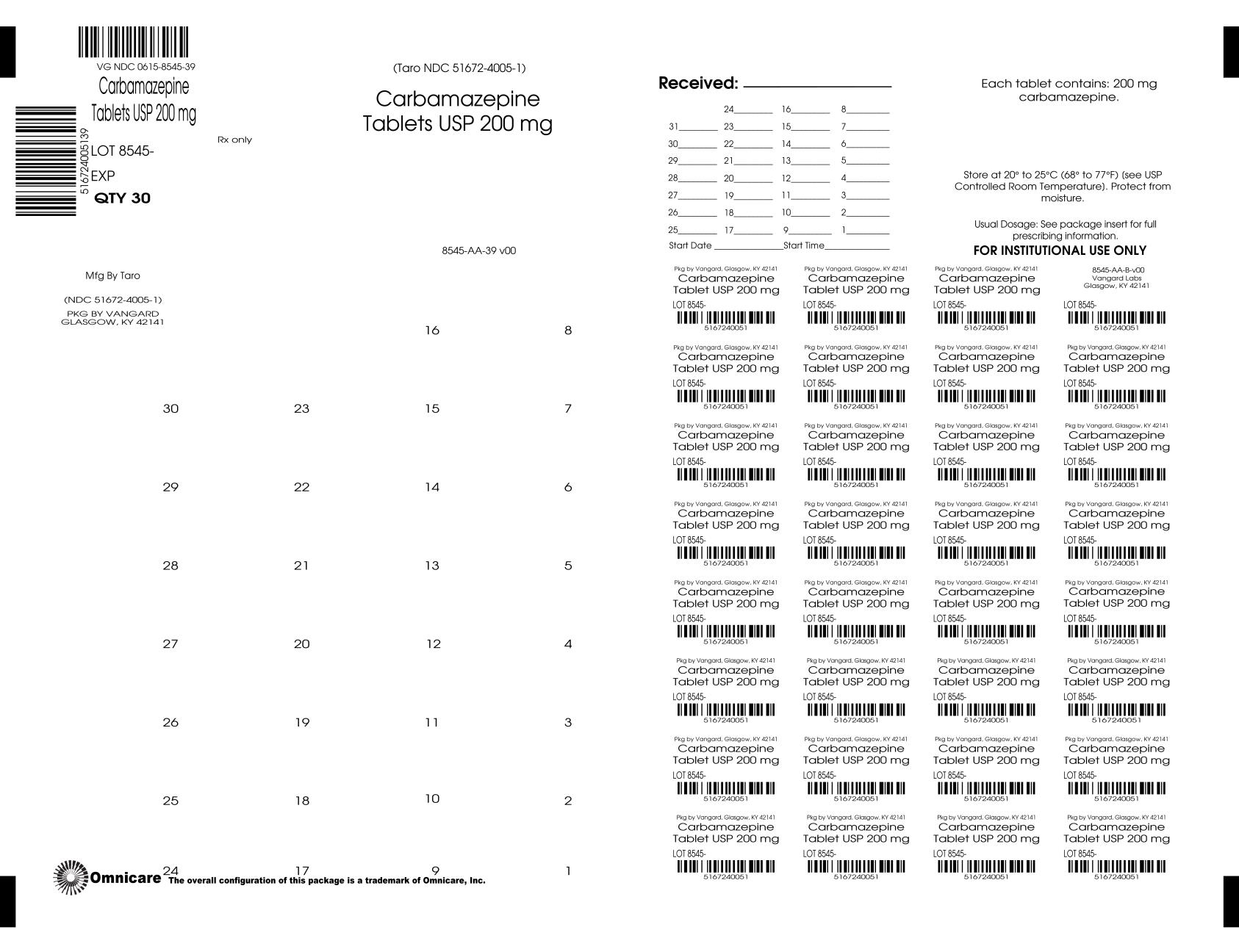
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information