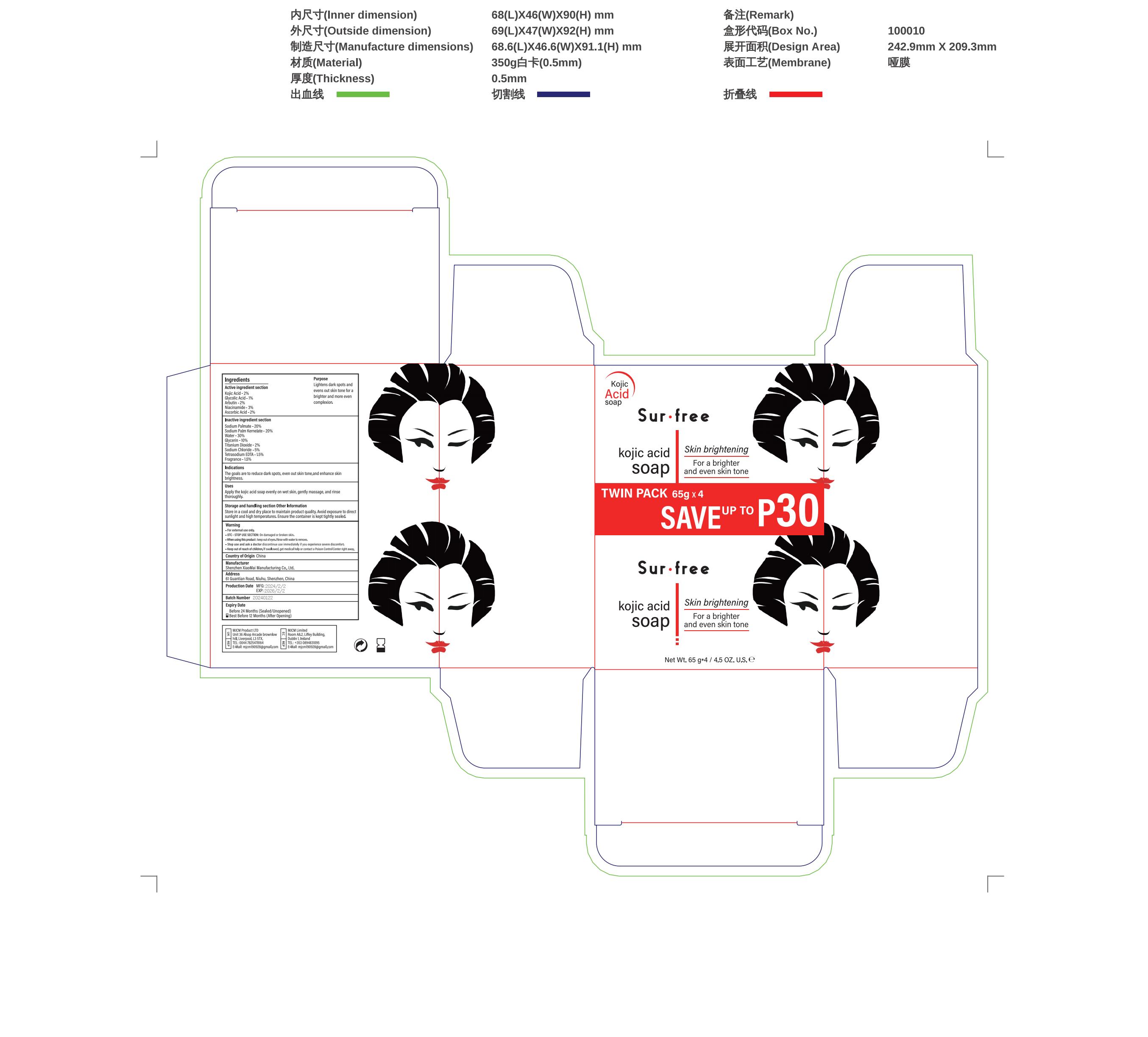
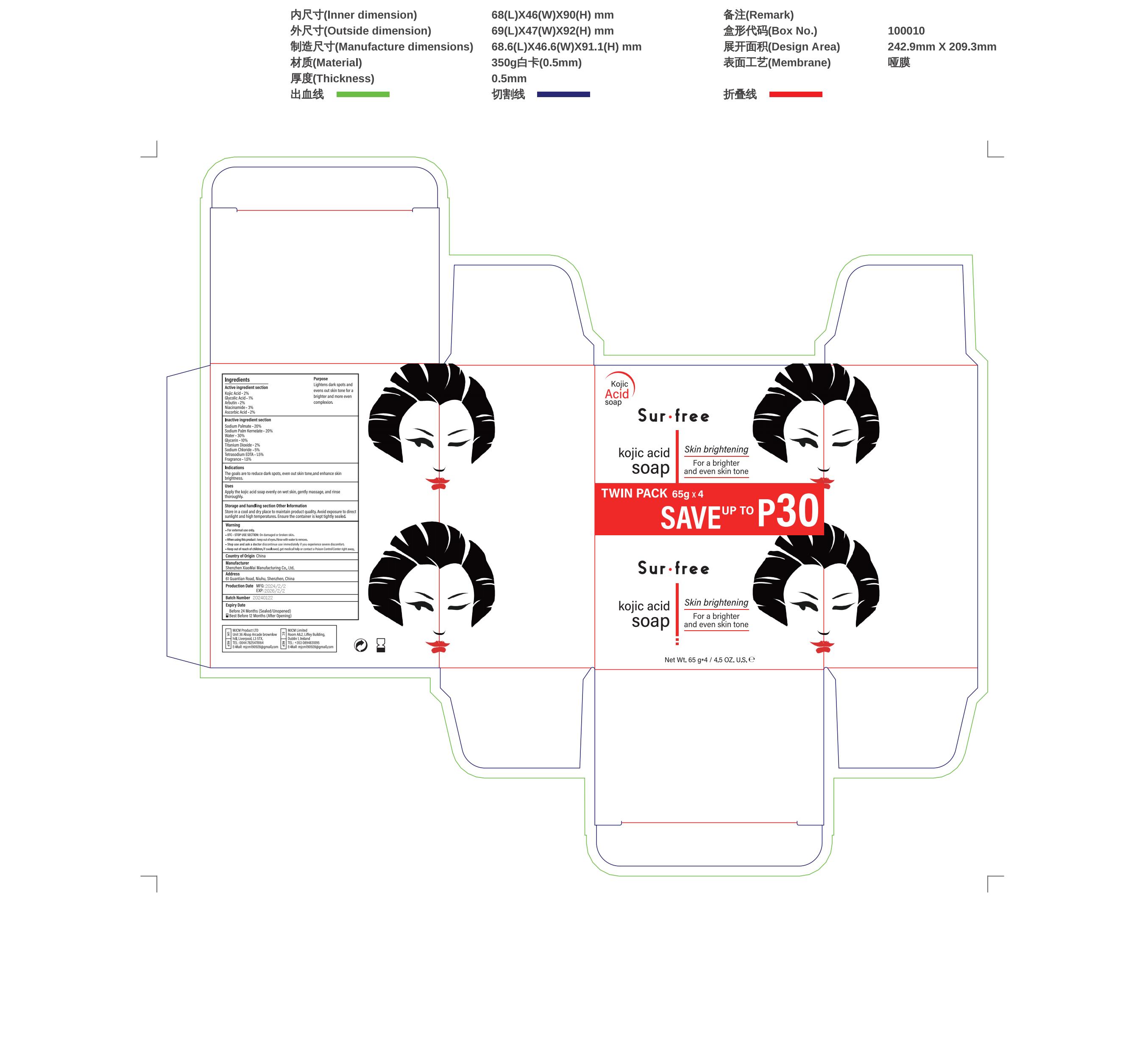
Label: KOJIC ACID- salicylic acid soap soap
- NDC Code(s): 83872-018-01
- Packager: Shenzhen XiaoMai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warning
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Inactive ingredients section
- Other information
- Directions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KOJIC ACID
salicylic acid soap soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOLIC ACID (UNII: 0WT12SX38S) (GLYCOLIC ACID - UNII:0WT12SX38S) GLYCOLIC ACID 1 g in 100 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 2 g in 100 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 3 g in 100 mg KOJIC ACID (UNII: 6K23F1TT52) (KOJIC ACID - UNII:6K23F1TT52) KOJIC ACID 2 g in 100 mg ARBUTIN (UNII: C5INA23HXF) (ARBUTIN - UNII:C5INA23HXF) ARBUTIN 2 g in 100 mg Inactive Ingredients Ingredient Name Strength SODIUM PALMATE (UNII: S0A6004K3Z) 20 g in 100 mg SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) 20 g in 100 mg DIGLYCERIN (UNII: 3YC120743U) 10 g in 100 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-018-01 260 mg in 1 BOX; Type 0: Not a Combination Product 02/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/17/2024 Labeler - Shenzhen XiaoMai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen XiaoMai Manufacturing Co., Ltd. 712999147 manufacture(83872-018)