Label: OPTASE- glycerin solution/ drops
- NDC Code(s): 72972-007-05, 72972-007-30
- Packager: Scope Health Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
WARNINGS
Warning
• For external use only.
• To avoid contamination, do not touch tip of container to any surface.
• If solution changes color or becomes cloudy, do not use.
• Do not use a damaged or punctured single-dose container.
• Wait at least 15 minutes between possible applications of other ophthalmic medication.
• Use immediately once opened.
• Do not reuse. Discard any remaining solution after use.
• Do not use if you are sensitive to any of the ingredients.
• Do not use after the expiry date shown on the pack.
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
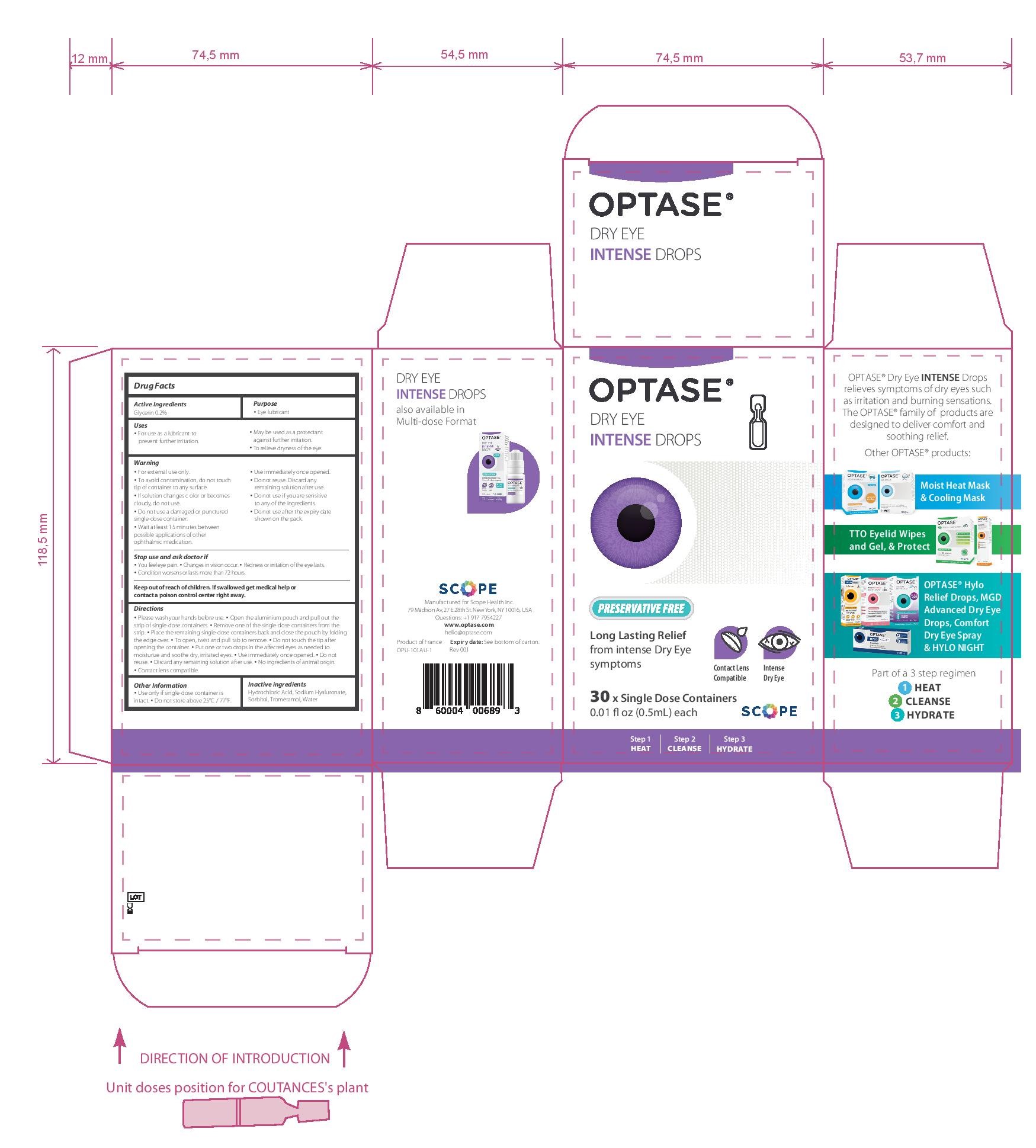
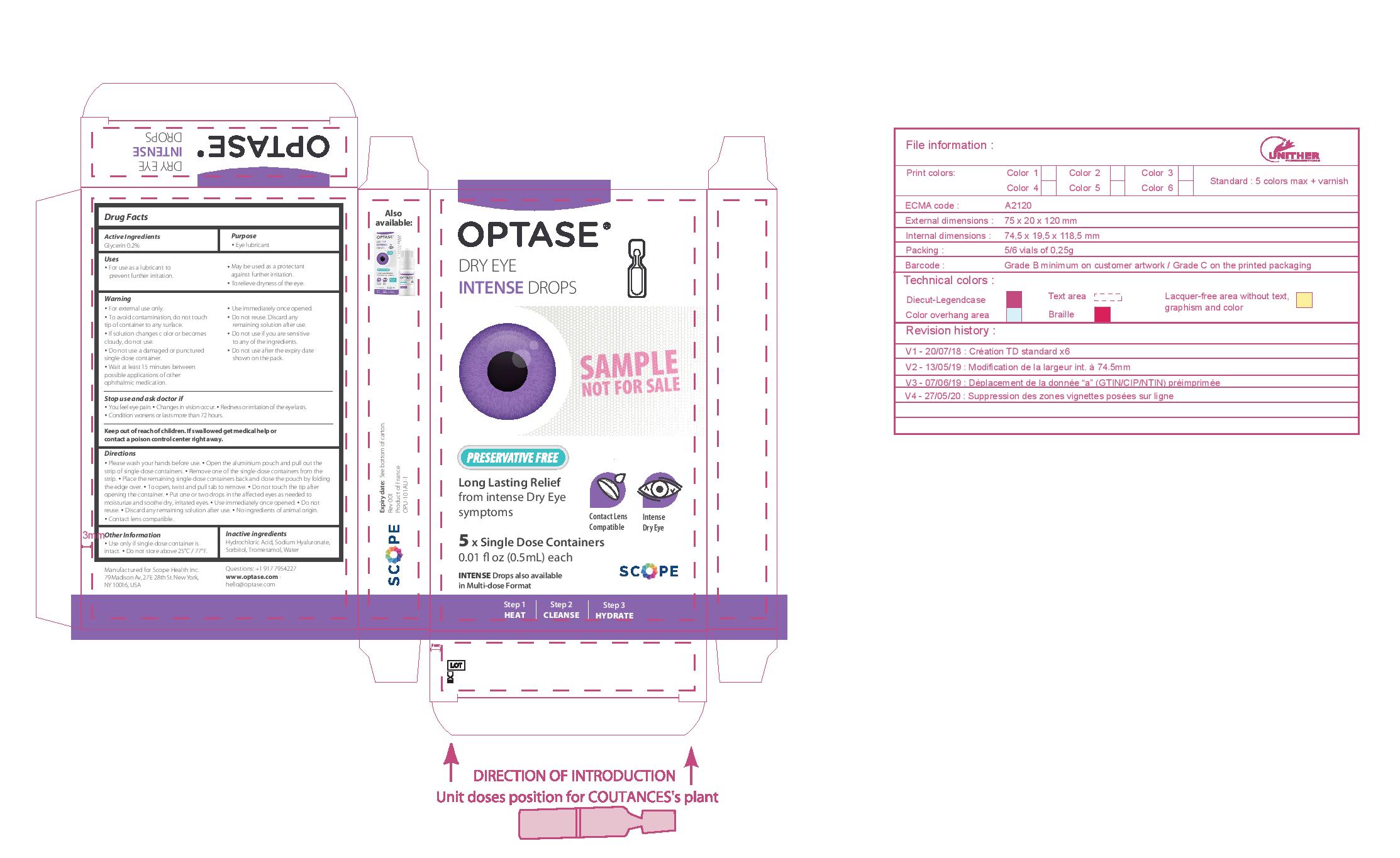
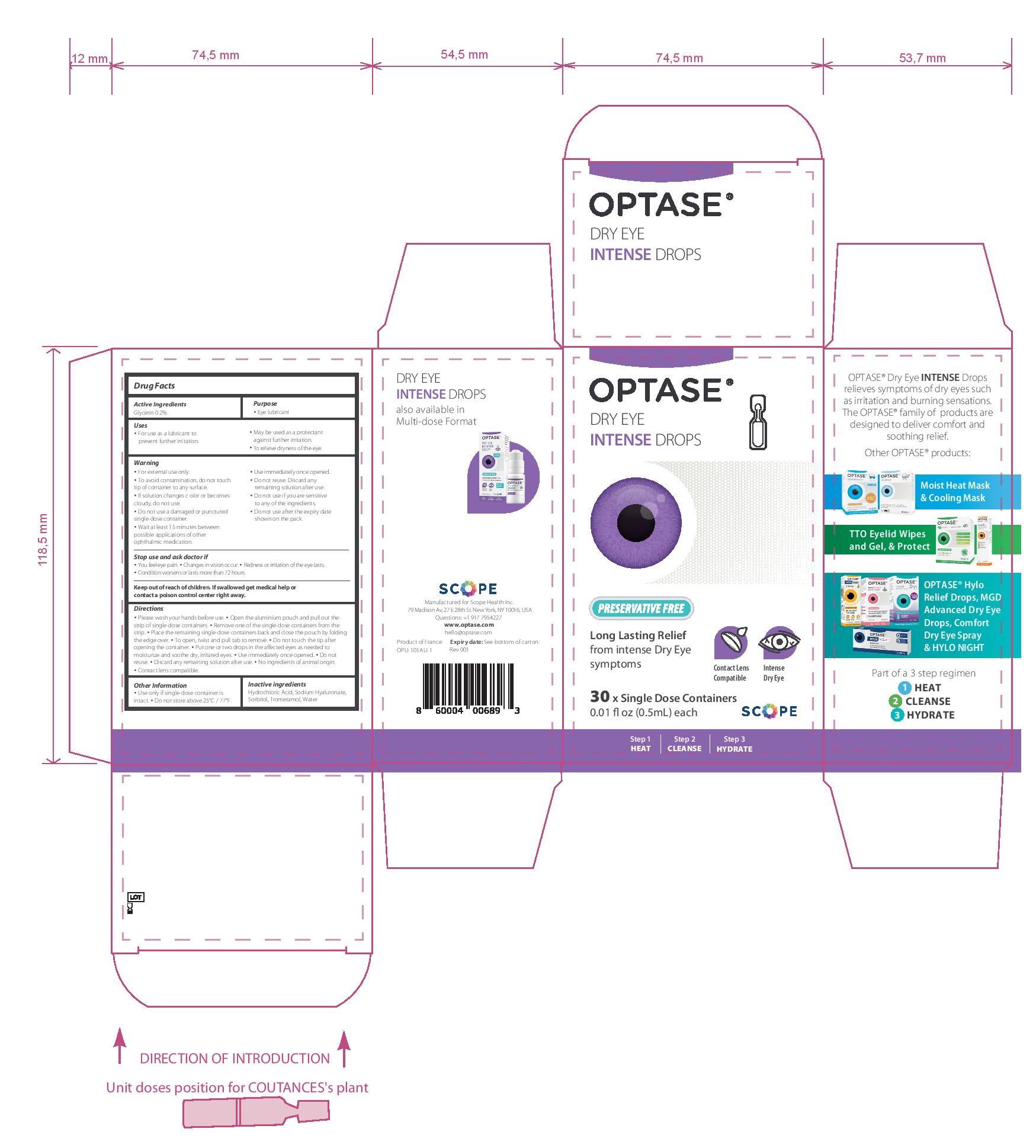
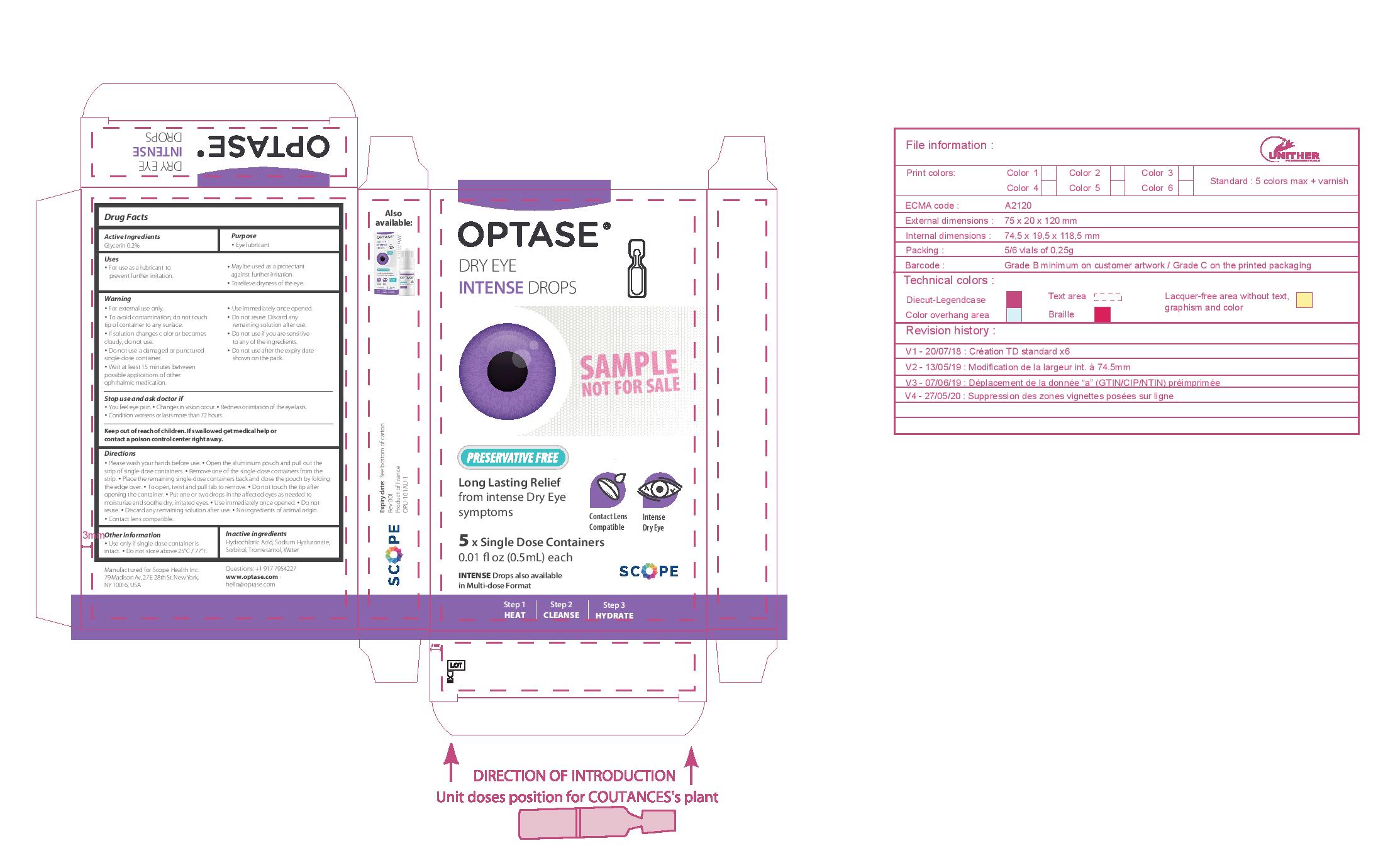
• Please wash your hands before use. • Open the aluminium pouch and pull out the strip of single-dose containers. • Remove one of the single-dose containers from the strip. • Place the remaining single-dose containers back and close the pouch by folding the edge over. • To open, twist and pull tab to remove. • Do not touch the tip after opening the container. • Put one or two drops in the affected eyes as needed • Use immediately once opened. • Do not reuse. • Discard any remaining solution after use.
• No ingredients of animal origin. • Contact lens compatible.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OPTASE
glycerin solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72972-007 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) TROMETHAMINE (UNII: 023C2WHX2V) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72972-007-30 1 in 1 BOX 02/07/2024 1 30 in 1 DOSE PACK 1 0.5 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:72972-007-05 1 in 1 BOX 02/07/2024 2 5 in 1 DOSE PACK 2 0.5 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/07/2024 Labeler - Scope Health Inc (116778693) Registrant - Scope Health Inc (116778693)