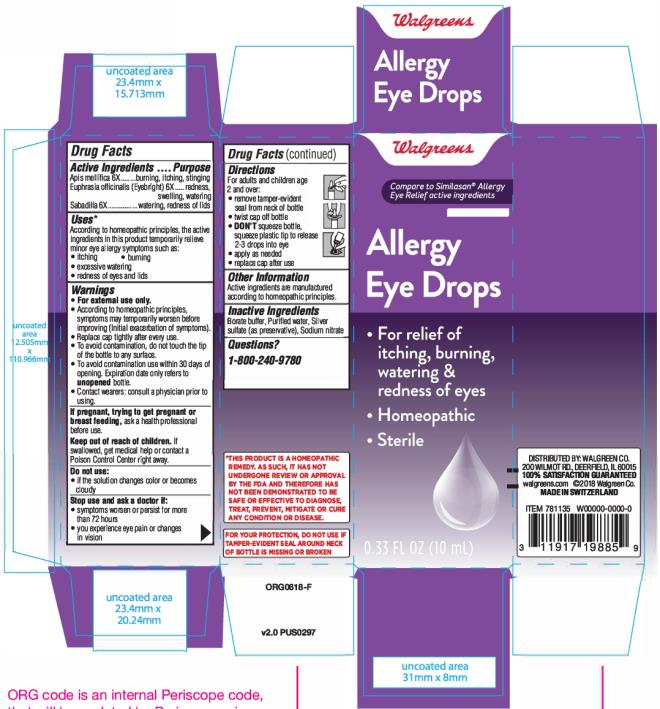
Label: WALGREENS ALLERGY EYE- apis mellifera, euphrasia stricta and schoenocaulon officinale seed solution/ drops
- NDC Code(s): 53799-400-11
- Packager: Similasan AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses*
-
Warnings
• For external use only.
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
• Replace cap tightly after every use.
• To avoid contamination, do not touch the tip of the bottle to any surface.
• To avoid contamination use within 30 days of opening. Expiration date only refers to unopened bottle.
• Contact wearers: consult a physician prior to using.
- Directions
- Other Information
- Inactive Ingredients
-
Questions?
1-800-240-9780
FOR YOUR PROTECTION DO NOT USE IF TAMPER EVIDENT SEAL AROUND NECK OF BOTTLE IS MISSING OR BROKEN
*THIS PRODUCT IS A HOMEOPATHIC REMEDY, AS SUCH, IT HAS NOT UNDERGONE REVIEW OR APPROVAL BY THE FDA AND THEREFORE HAS NOT BEEN DEMONSTRATED TO BE SAFE OR EFFECTIVE TO DIAGNOSE, TREAT, PREVENT, MITIGATE OR CURE ANY CONDITION OR DISEASE.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WALGREENS ALLERGY EYE
apis mellifera, euphrasia stricta and schoenocaulon officinale seed solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53799-400 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 0.45 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 0.45 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 6 [hp_X] in 0.45 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SILVER SULFATE (UNII: 8QG6HV4ZPO) SODIUM NITRATE (UNII: 8M4L3H2ZVZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53799-400-11 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 06/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2018 Labeler - Similasan AG (481545754) Registrant - Similasan Corporation (111566530)