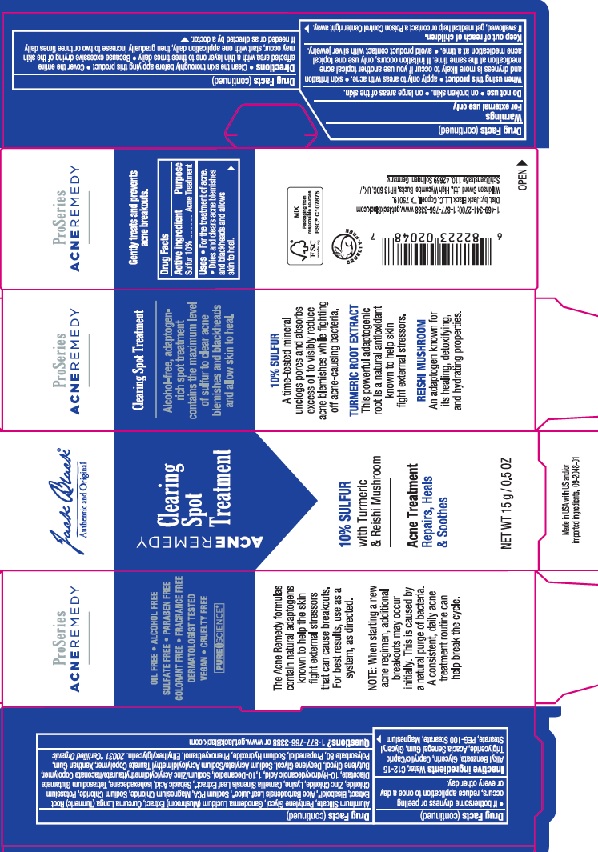
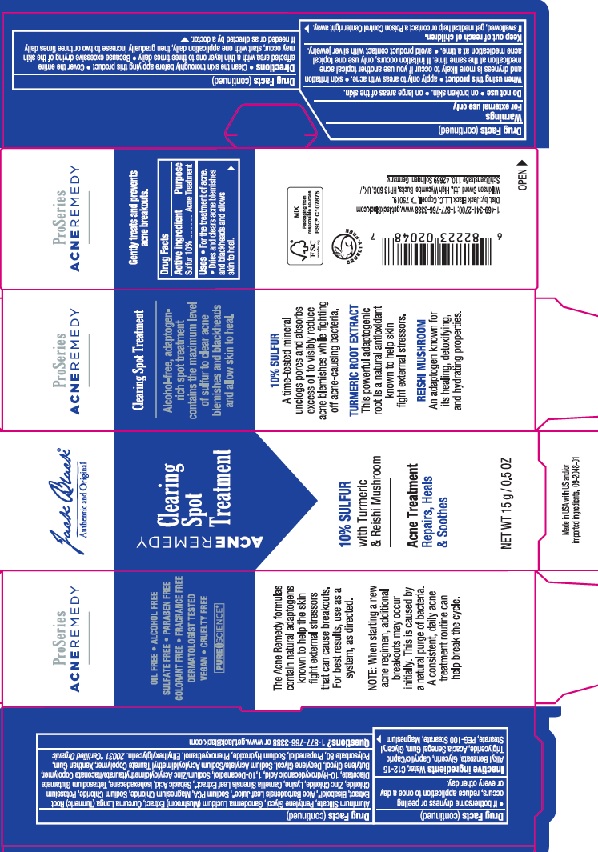
Label: JACK BLACK CLEARING SPOT TREATMENT- sulfur gel
- NDC Code(s): 66738-436-01
- Packager: Jack Black LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Water, C12-15 Alkyl Benzoate, Glycerin, Caprylic/Capric Triglyceride, Acacia Senegal Gum, Magnesium Aluminum Silicate, Glyceryl Stearate,
PEG-100 Stearate, Pentylene Glycol, Phenoxyethanol, Butylene Glycol, Decylene Glycol, Zinc PCA, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Xanthan Gum, Sodium PCA, Isohexadecane, Ganoderma Lucidum Extract, Sodium Hydroxide, Bisabolol, Ethylhexylglycerin, Polysorbate 80, Lysine, Propanediol, Aloe Barbadensis Leaf Juice, Tetrasodium Glutamate Diacetate, Curcuma Longa (Turmeric) Root Extract, Camellia Sinensis Leaf Extract, 10-Hydroxydecanoic Acid, Sebacic Acid, Magnesium Chloride, Potassium Chloride,
Sodium Chloride, 1,10-Decanediol, Zinc Chloride - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JACK BLACK CLEARING SPOT TREATMENT
sulfur gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66738-436 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CURCUMA LONGA WHOLE (UNII: W5488JUO8U) CAMELLIA SINENSIS FLOWER (UNII: 9I2BJY2J17) ALOE (UNII: V5VD430YW9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ACACIA SENEGAL FLOWER (UNII: 72P931MTC2) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PENTYLENE GLYCOL (UNII: 50C1307PZG) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PHENOXYETHANOL (UNII: HIE492ZZ3T) DECYLENE GLYCOL (UNII: S57M60MI88) ZINC PIDOLATE (UNII: C32PQ86DH4) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) ISOHEXADECANE (UNII: 918X1OUF1E) GANODERMA LUCIDUM WHOLE (UNII: J5P04QW0CF) SODIUM HYDROXIDE (UNII: 55X04QC32I) LEVOMENOL (UNII: 24WE03BX2T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LYSINE (UNII: K3Z4F929H6) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) 10-HYDROXYDECANOIC ACID (UNII: NP03XO416B) SEBACIC ACID (UNII: 97AN39ICTC) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM CHLORIDE (UNII: 451W47IQ8X) 1,10-DECANEDIOL (UNII: 5I577UDK52) ZINC CHLORIDE (UNII: 86Q357L16B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66738-436-01 1 in 1 CARTON 02/12/2021 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 02/12/2021 Labeler - Jack Black LLC (847024036)