Label: 10% POVIDONE IODINE NASAL SWAB- povidone iodine nasal swab solution
- NDC Code(s): 83062-497-26
- Packager: IIMED MEDICAL MEXICANA S DE RL DE CV
- This is a repackaged label.
- Source NDC Code(s): 53329-497
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
NASAL APPLICATION:

- Use a tissue to clean the inside of both nostrils, including the inside tip of nostril. Discard.
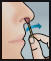
- Insert swab comfortably into one nostril and rotate for 30 seconds, covering all surfaces. Discard swabstick.
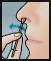
- Using a 2nd swab, repeat step 2 with the other nostril.
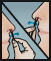
- Repeat the application in both nostrils, using the 3rd and 4th swabs.
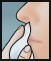
- Do not blow nose. If solution drips, gently wipe with a tissue.
- Other information
- Inactive ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
10% POVIDONE IODINE NASAL SWAB
povidone iodine nasal swab solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83062-497(NDC:53329-497) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) NONOXYNOL-10 (UNII: K7O76887AP) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83062-497-26 10 mL in 1 PACKET; Type 0: Not a Combination Product 10/15/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/15/2023 Labeler - IIMED MEDICAL MEXICANA S DE RL DE CV (812894376) Registrant - Medline Industries, LP (025460908) Establishment Name Address ID/FEI Business Operations IIMED MEDICAL MEXICANA S DE RL DE CV 812894376 relabel(83062-497) , repack(83062-497)