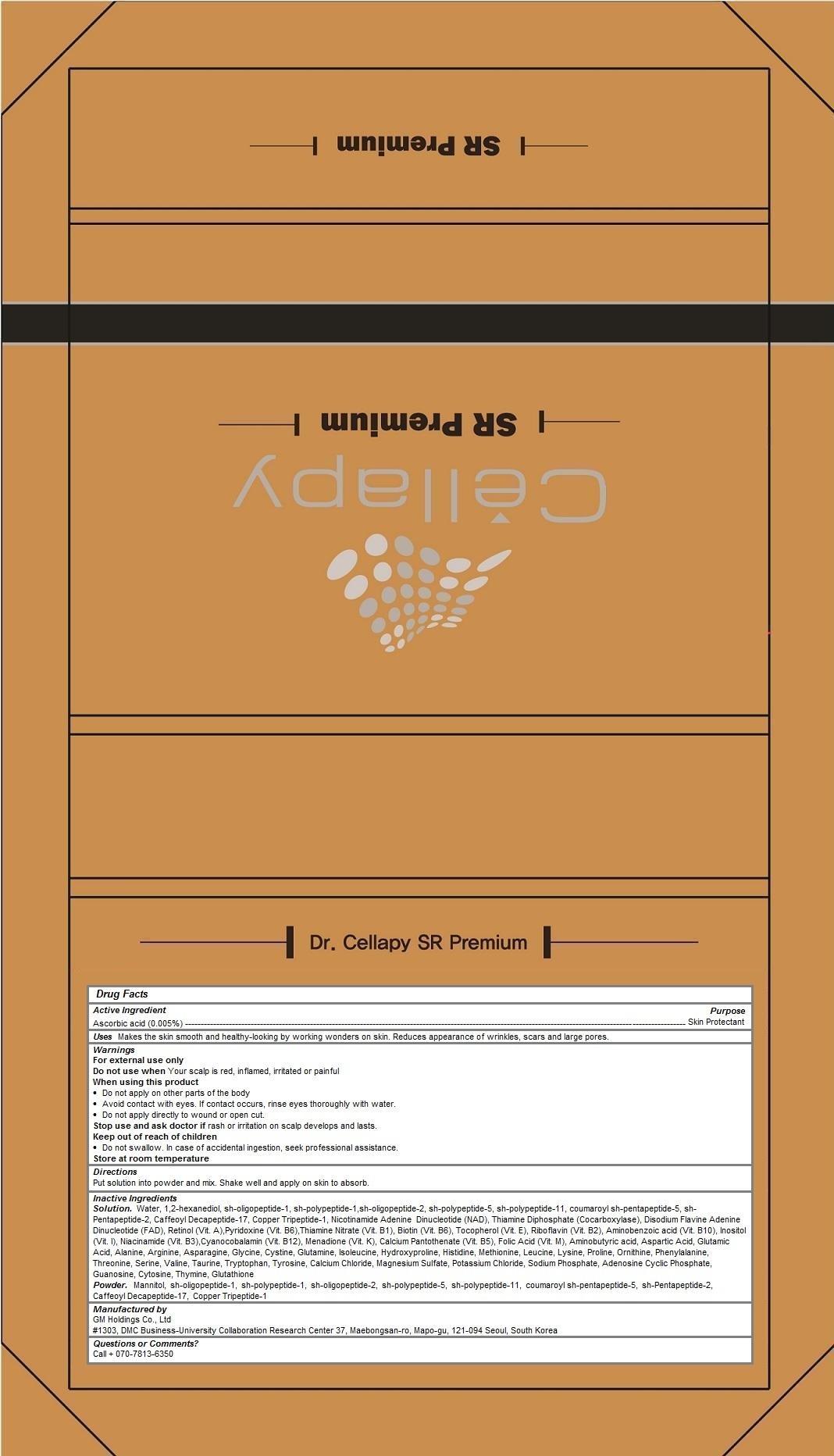
Label: DR CELLAPY SR PREMIUM- ascorbic acid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 69278-100-02, 69278-101-01, 69278-101-02, 69278-102-01, view more69278-102-02 - Packager: GM Holdings Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Dr. Cellapy SR Premium
Makes the skin smooth and healthy-looking by working wonders on skin. Reduces appearance of wrinkles, scars and large pores.
For external use only
Do not use when Your scalp is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on scalp develops and lasts.
Store at room temperatureSolution. Water, 1,2-hexanediol, sh-oligopeptide-1, sh-polypeptide-1,sh-oligopeptide-2, sh-polypeptide-5, sh-polypeptide-11, coumaroyl sh-pentapeptide-5, sh-Pentapeptide-2, Caffeoyl Decapeptide-17, Copper Tripeptide-1, Nicotinamide Adenine Dinucleotide (NAD), Thiamine Diphosphate (Cocarboxylase), Disodium Flavine Adenine Dinucleotide (FAD), Retinol (Vit. A),Pyridoxine (Vit. B6),Thiamine Nitrate (Vit. B1), Biotin (Vit. B6), Tocopherol (Vit. E), Riboflavin (Vit. B2), Aminobenzoic acid (Vit. B10), Inositol (Vit. I), Niacinamide (Vit. B3),Cyanocobalamin (Vit. B12), Menadione (Vit. K), Calcium Pantothenate (Vit. B5), Folic Acid (Vit. M), Aminobutyric acid, Aspartic Acid, Glutamic Acid, Alanine, Arginine, Asparagine, Glycine, Cystine, Glutamine, Isoleucine, Hydroxyproline, Histidine, Methionine, Leucine, Lysine, Proline, Ornithine, Phenylalanine, Threonine, Serine, Valine, Taurine, Tryptophan, Tyrosine, Calcium Chloride, Magnesium Sulfate, Potassium Chloride, Sodium Phosphate, Adenosine Cyclic Phosphate, Guanosine, Cytosine, Thymine, Glutathione
Powder. Mannitol, sh-oligopeptide-1, sh-polypeptide-1, sh-oligopeptide-2, sh-polypeptide-5, sh-polypeptide-11, coumaroyl sh-pentapeptide-5, sh-Pentapeptide-2, Caffeoyl Decapeptide-17, Copper Tripeptide-1 -
INGREDIENTS AND APPEARANCE
DR CELLAPY SR PREMIUM
ascorbic acid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69278-100 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69278-100-02 1 in 1 BOX 01/20/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 5 mL Part 2 1 VIAL 200 mg Part 1 of 2 DR. CELLAPY SR PREMIUM SOLUTION
ascorbic acid liquidProduct Information Item Code (Source) NDC:69278-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 0.005 in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) NEPIDERMIN (UNII: TZK30RF92W) BASIC FIBROBLAST GROWTH FACTOR (HUMAN) (UNII: S3529G9M9V) MECASERMIN (UNII: 7GR9I2683O) FIBROBLAST GROWTH FACTOR-1 (UNII: G53298VN9Y) PREZATIDE COPPER (UNII: 6BJQ43T1I9) NADIDE (UNII: 0U46U6E8UK) COCARBOXYLASE (UNII: Q57971654Y) FLAVIN ADENINE DINUCLEOTIDE (UNII: ZC44YTI8KK) RETINOL (UNII: G2SH0XKK91) PYRIDOXINE (UNII: KV2JZ1BI6Z) THIAMINE MONONITRATE (UNII: 8K0I04919X) BIOTIN (UNII: 6SO6U10H04) TOCOPHEROL (UNII: R0ZB2556P8) RIBOFLAVIN (UNII: TLM2976OFR) AMINOBENZOIC ACID (UNII: TL2TJE8QTX) INOSITOL (UNII: 4L6452S749) NIACINAMIDE (UNII: 25X51I8RD4) CYANOCOBALAMIN (UNII: P6YC3EG204) MENADIONE (UNII: 723JX6CXY5) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) FOLIC ACID (UNII: 935E97BOY8) .GAMMA.-AMINOBUTYRIC ACID (UNII: 2ACZ6IPC6I) ASPARTIC ACID (UNII: 30KYC7MIAI) GLUTAMIC ACID (UNII: 3KX376GY7L) ALANINE (UNII: OF5P57N2ZX) ARGININE (UNII: 94ZLA3W45F) ASPARAGINE (UNII: 5Z33R5TKO7) GLYCINE (UNII: TE7660XO1C) CYSTINE (UNII: 48TCX9A1VT) GLUTAMINE (UNII: 0RH81L854J) ISOLEUCINE (UNII: 04Y7590D77) HYDROXYPROLINE (UNII: RMB44WO89X) HISTIDINE (UNII: 4QD397987E) METHIONINE (UNII: AE28F7PNPL) LEUCINE (UNII: GMW67QNF9C) LYSINE (UNII: K3Z4F929H6) PROLINE (UNII: 9DLQ4CIU6V) ORNITHINE (UNII: E524N2IXA3) PHENYLALANINE (UNII: 47E5O17Y3R) THREONINE (UNII: 2ZD004190S) SERINE (UNII: 452VLY9402) VALINE (UNII: HG18B9YRS7) TAURINE (UNII: 1EQV5MLY3D) TRYPTOPHAN (UNII: 8DUH1N11BX) TYROSINE (UNII: 42HK56048U) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM PHOSPHATE (UNII: SE337SVY37) ADENOSINE CYCLIC PHOSPHATE (UNII: E0399OZS9N) GUANOSINE (UNII: 12133JR80S) CYTOSINE (UNII: 8J337D1HZY) THYMINE (UNII: QR26YLT7LT) GLUTATHIONE (UNII: GAN16C9B8O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69278-101-02 5 in 1 BOX 1 NDC:69278-101-01 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/10/2014 Part 2 of 2 DR CELLAPY SR PREMIUM POWDER
face powders liquidProduct Information Item Code (Source) NHRIC:69278-102 Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR MANNITOL (UNII: 3OWL53L36A) INGR NEPIDERMIN (UNII: TZK30RF92W) INGR BASIC FIBROBLAST GROWTH FACTOR (HUMAN) (UNII: S3529G9M9V) INGR MECASERMIN (UNII: 7GR9I2683O) INGR FIBROBLAST GROWTH FACTOR-1 (UNII: G53298VN9Y) INGR PREZATIDE COPPER (UNII: 6BJQ43T1I9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69278-102-02 5 in 1 BOX 1 NHRIC:69278-102-01 200 mg in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/10/2014 Labeler - GM Holdings Co., Ltd (688439958) Registrant - GM Holdings Co., Ltd (688439958) Establishment Name Address ID/FEI Business Operations GM Holdings Co., Ltd 688439958 manufacture(69278-100)