Label: MEMBERS MARK- chloroxylenol solution
- NDC Code(s): 47593-573-11
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
-
INACTIVE INGREDIENT
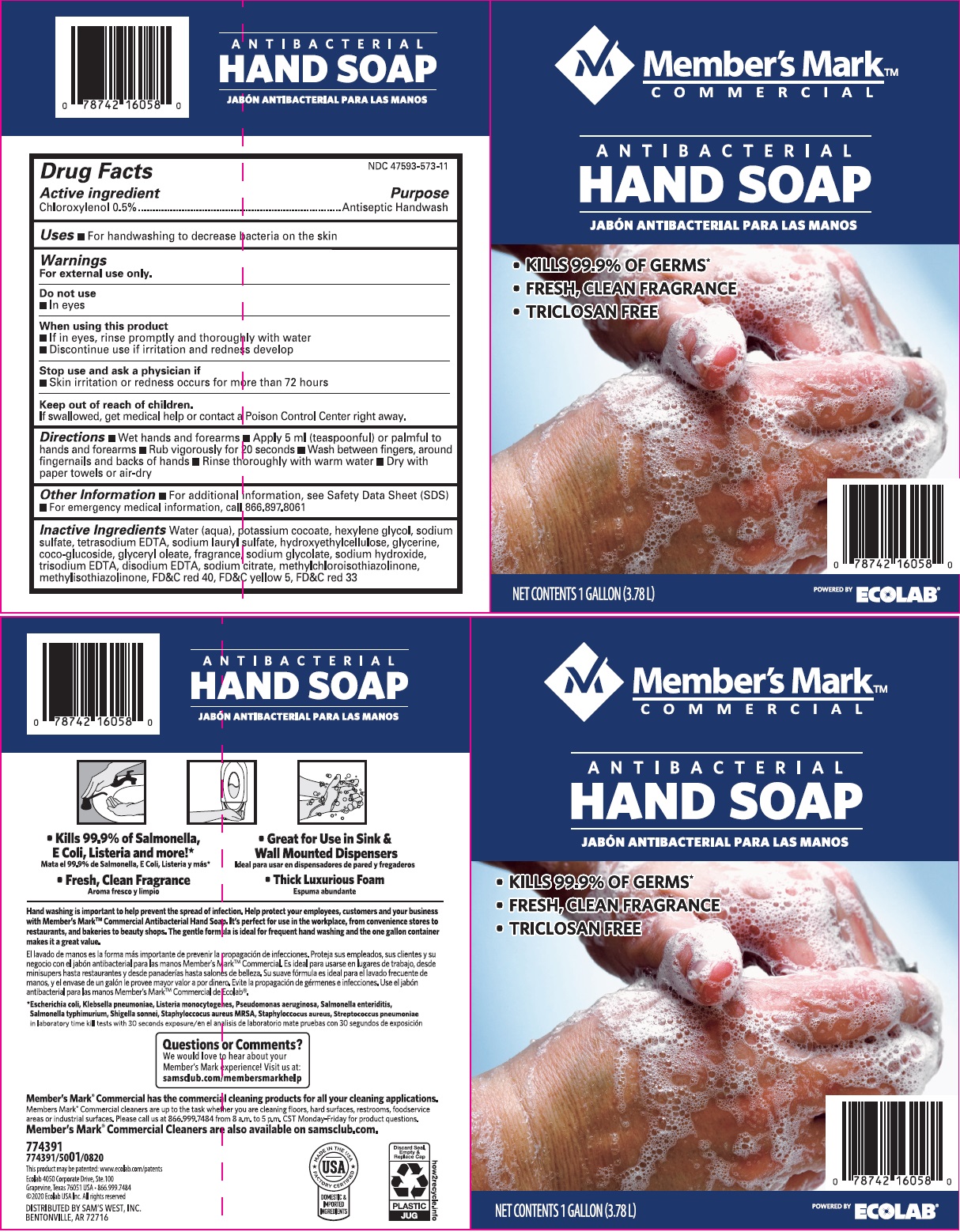
Inactive Ingredients Water (Aqua), Potassium Cocoate, Hexylene Glycol, Sodium Sulfate, Tetrasodium EDTA, Sodium lauryl sulfate, Hydroxyethylcellulose, Glycerin, Coco-glucoside, Glyceryl Oleate, fragrance, sodium glycolate, Sodium Hydroxide, Trisodium EDTA, Disodium EDTA, Sodium Citrate, Methylchloroisothiazolinone, Methylisothiazolinone, Red 40, Yellow 5, Red 33
-
Principal display panel and representative label
Member's Mark
COMMERCIAL
ANTIBACTERIAL
HAND SOAP
- KILLS 99.9% OF GERMS*
- FRESH, CLEAN FRAGRANCE
- TRICLOSAN FREE
NET CONTENTS 1 GALLON (3.78 L)
Ecolab 4050 Corporate Drive, Ste. 100
Grapevine, Texas 76051 USA - 866.999.7484
(c) 2020 Ecolab USA Inc. All rights reserved
DISTRIBUTED BY SAM'S WEST, INC.
BENTONVILLE, AR 72716
-
INGREDIENTS AND APPEARANCE
MEMBERS MARK
chloroxylenol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-573 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM SULFATE (UNII: 0YPR65R21J) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) GLYCERIN (UNII: PDC6A3C0OX) COCO GLUCOSIDE (UNII: ICS790225B) GLYCERYL OLEATE (UNII: 4PC054V79P) SODIUM GLYCOLATE (UNII: B75E535IMI) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE TRISODIUM (UNII: 420IP921MB) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CITRATE (UNII: 1Q73Q2JULR) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-573-11 3780 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/28/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 07/28/2016 Labeler - Ecolab Inc. (006154611)

