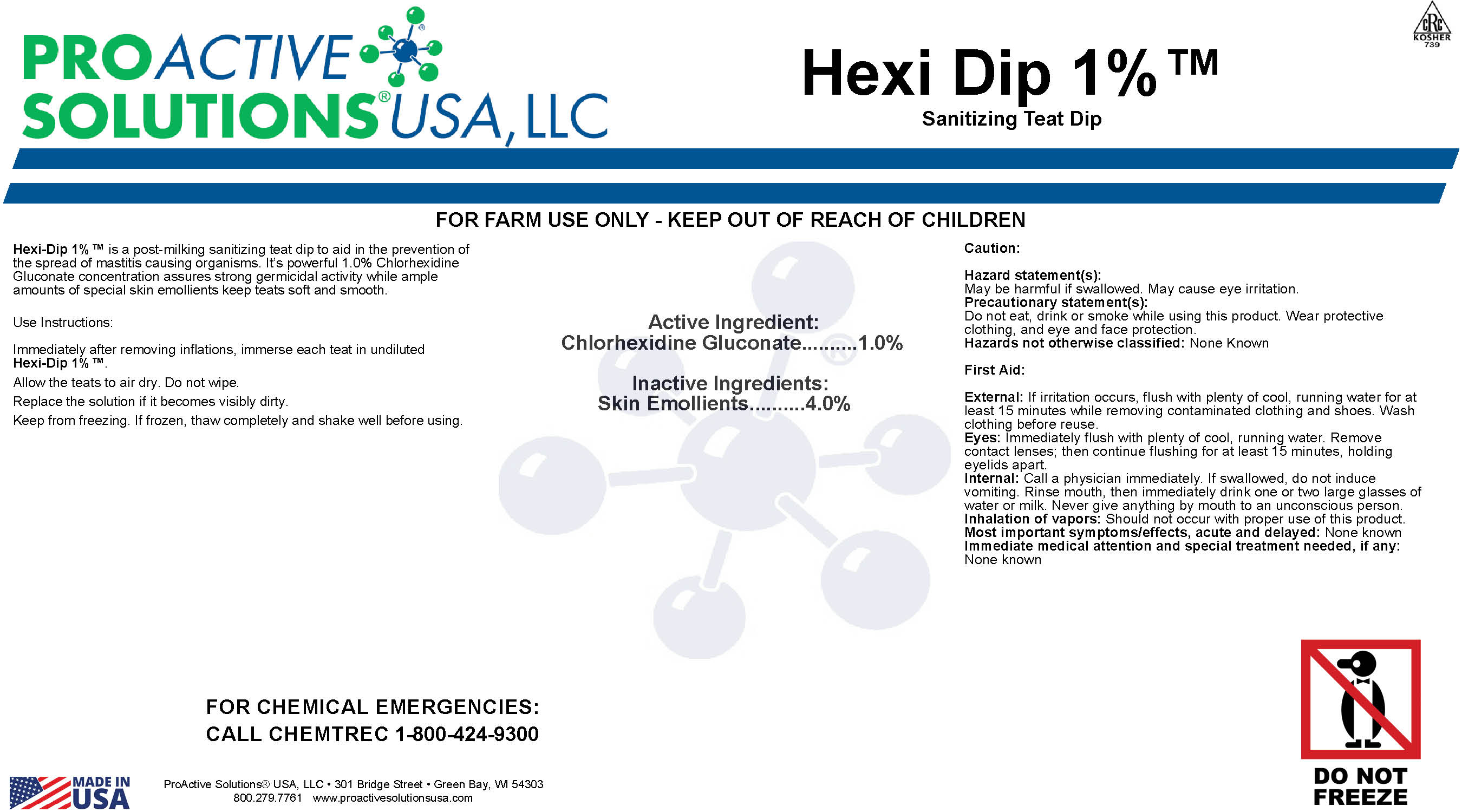
Label: HEXI-DIP 1%- chlorhexidine gluconate liquid
-
NDC Code(s):
63927-4185-1,
63927-4185-2,
63927-4185-3,
63927-4185-4, view more63927-4185-5
- Packager: ProActive Solutions USA, LLC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Hexi-Dip 1%
Hexi-Dip 1%™ is a post-milking sanitizing teat dip to aid in the prevention of the spread of
mastitis causing organisms. It’s powerful 1.0% Chlorhexidine Gluconate concentration
assures strong germicidal activity while ample amounts of special skin emollients
keep teats soft and smooth.Use instructions:
Immediately after removing inflations, immerse each teat in undiluted
Hexi-Dip 1%™.Allow the teats to air dry. Do not wipe.
Replace the solution if it becomes visibly dirty.
Keep from freezing. If frozen, thaw completely and shake well before using.
Caution:
Hazard statement(s) May be harmful if swallowed. May cause eye irritation.
Precautionary statement(s):
Do not eat, drink or smoke while using this product. Wear protective clothing, and eye
and face protection.
Hazards not otherwise classified: None KnownFirst Aid:
External: If irritation occurs, flush with plenty of cool, running water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before
reuse.
Eyes: Immediately flush with plenty of cool, running water. Remove contact lenses;
then continue flushing for at least 15 minutes, holding eyelids apart.
Internal: Call a physician immediately. If swallowed, do not induce vomiting. Rinse
mouth, then immediately drink one or two large glasses of water or milk. Never give
anything by mouth to an unconscious person.
Inhalation of vapors: Should not occur with proper use of this product.
Most important symptoms/effects, acute and delayed: None known
Immediate medical attention and special treatment needed, if any: None known - Hexi-Dip 1%
-
INGREDIENTS AND APPEARANCE
HEXI-DIP 1%
chlorhexidine gluconate liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:63927-4185 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 1 kg in 100 kg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.0136 kg in 100 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63927-4185-1 15.28 kg in 1 DRUM 2 NDC:63927-4185-2 19.11 kg in 1 DRUM 3 NDC:63927-4185-3 57.31 kg in 1 DRUM 4 NDC:63927-4185-4 114.62 kg in 1 DRUM 5 NDC:63927-4185-5 210.14 kg in 1 DRUM Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/30/2009 Labeler - ProActive Solutions USA, LLC (029368225) Registrant - ProActive Solutions USA, LLC (029368225) Establishment Name Address ID/FEI Business Operations ProActive Solutions USA, LLC 029368225 api manufacture, manufacture, pack, repack, label