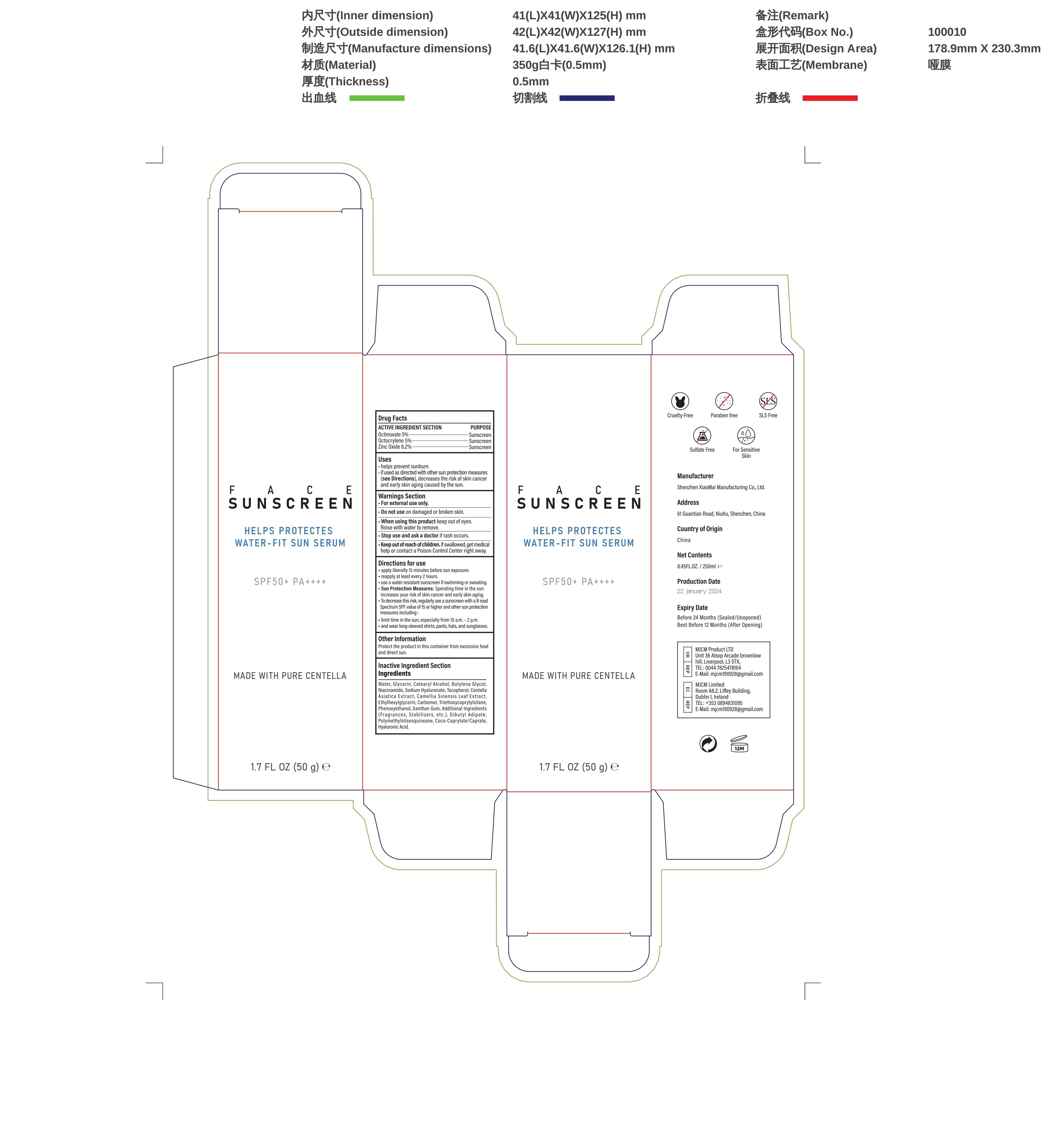
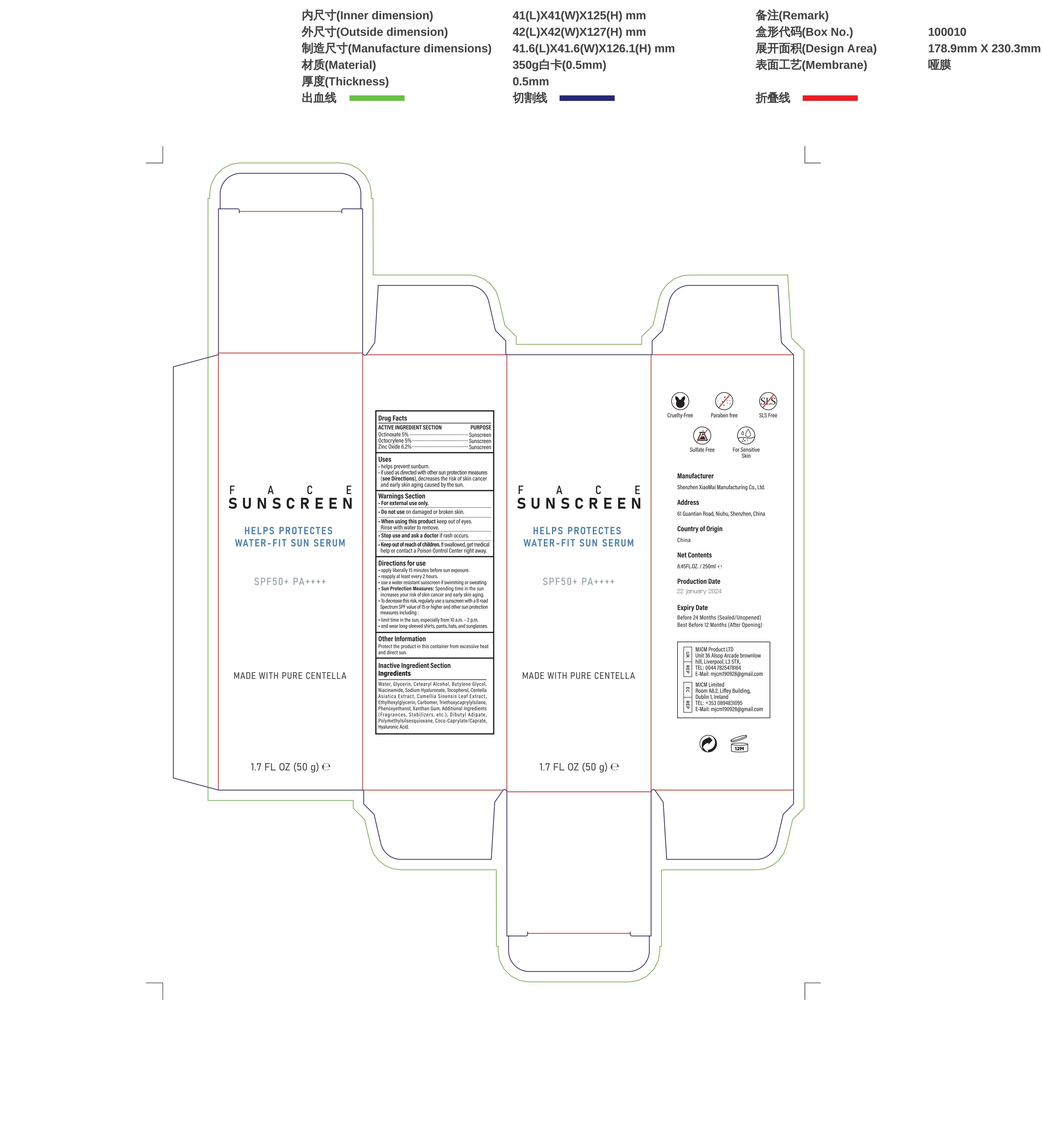
Label: FACE SUNSCREEN SKIN CARE- face sunscreen cream
- NDC Code(s): 83872-012-01
- Packager: Shenzhen Xiaomai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
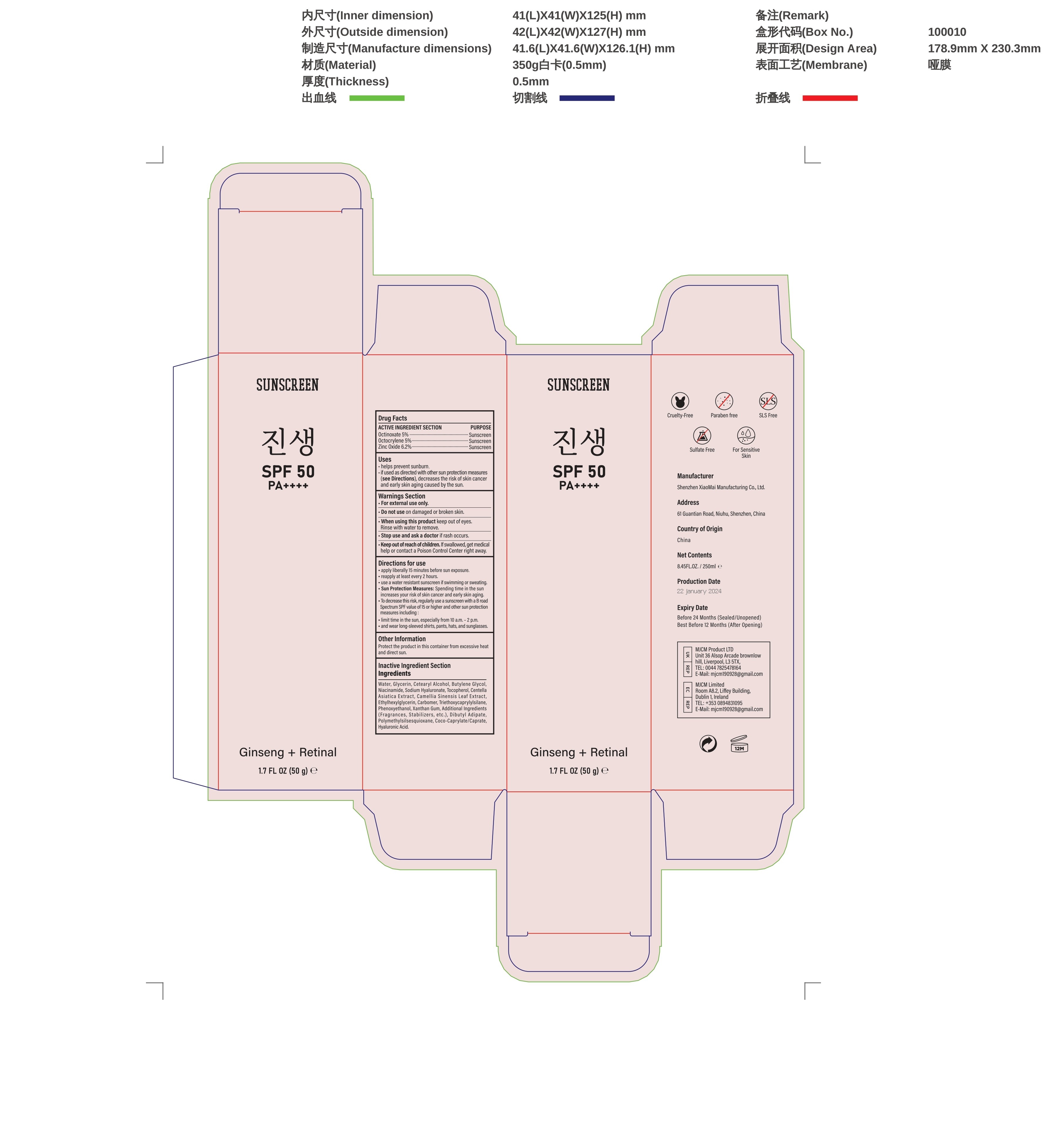
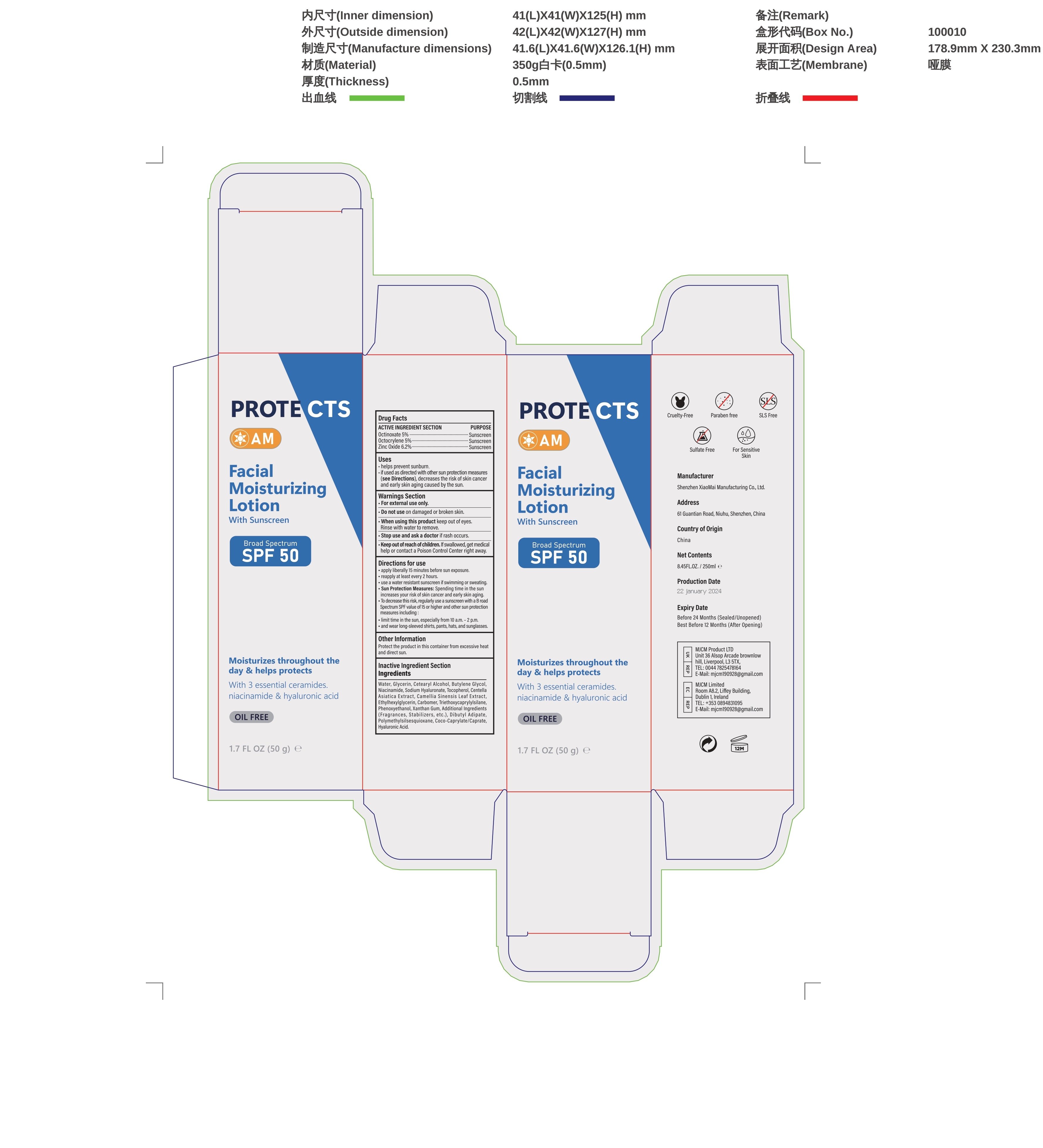
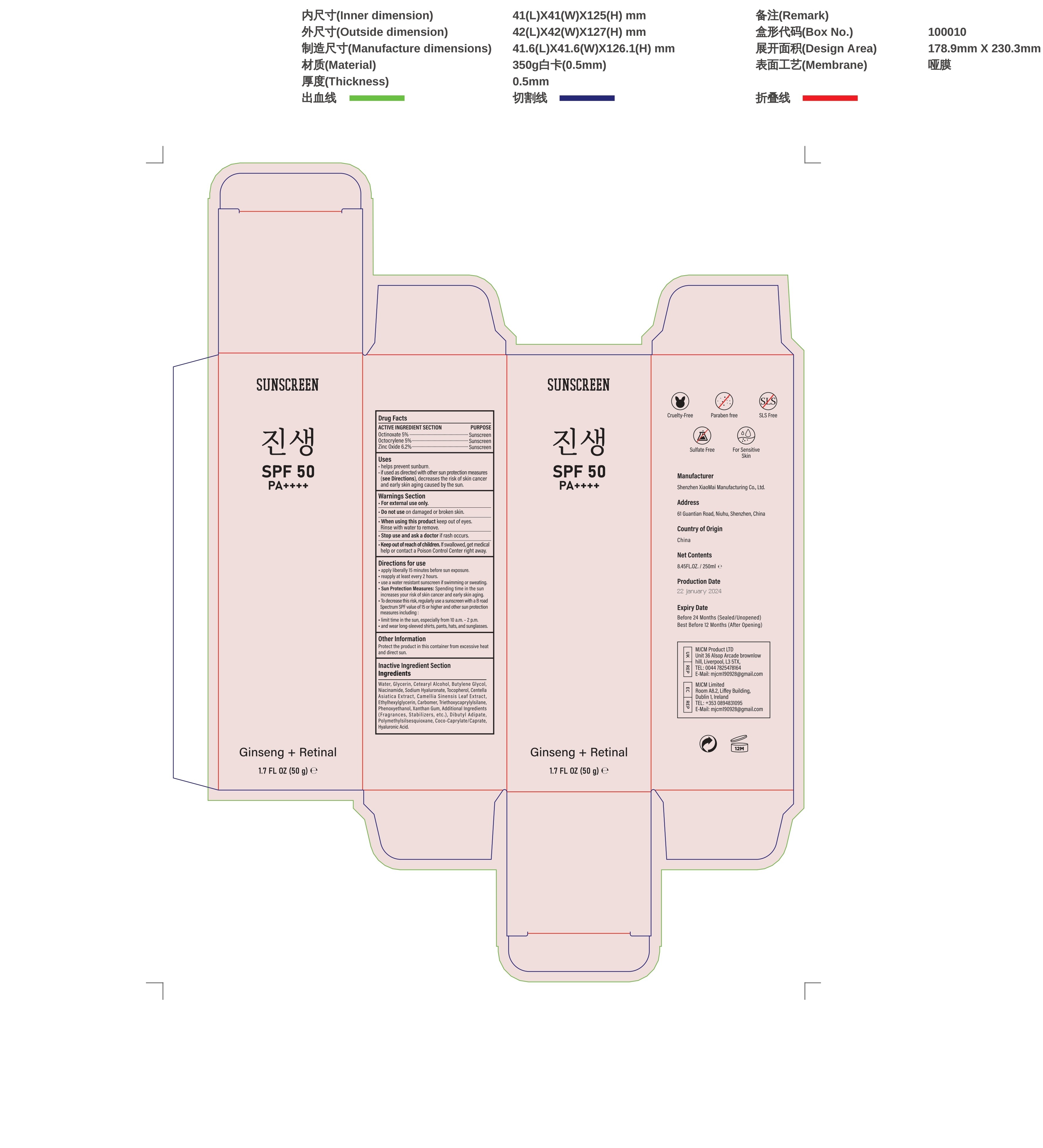
- Active ingredients
- Purpose
- Uses
- Wamings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Ask Doctor
-
Directions
Directions for use
-apply liberally 15 minutes before sun exposure.
-reapply at least every 2 hours.
-use a water resistant sunscreen if swimming or sweating.
-Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging
-To decrease this risk, regularly use a sunscreen with a B road.
-Spectrum SPF value of15 or higher and other sun protection:
-limit time in the sun, especially from 10 a.m.-2 p.m.
-and wear long-sleeved shirts, pants, hats, and sunglasses - Other information
-
Inactive ingredients
Water, Glycerin, Cetearyl Alcohol, Butylene Glycol,Niacinamide, Sodium Hyaluronate, Tocopherol, Centella,Asiatica Extract,Camellia Sinensis Leaf Extract,Ethylhexylglycerin, Carbomer, Triethoxycaprylyisilane,Phenoxyethanol,Xanthan Gum,Additional Ingredients(Fragrances,Stabilizers,etc.),Dibutyl Adipate,Polymethylsiisesquioxane,Coco-Caprylate/Caprate,Hyaluronic Acid.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FACE SUNSCREEN SKIN CARE
face sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.2 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) GLYCERIN (UNII: PDC6A3C0OX) HYALURONATE SODIUM (UNII: YSE9PPT4TH) NIACINAMIDE (UNII: 25X51I8RD4) CETEARYL ETHYLHEXANOATE (UNII: 9M64UO4C25) 1,3-BUTYLENE GLYCOL 1-PROPIONATE (UNII: 17U77WTV66) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-012-01 50 g in 1 BOTTLE; Type 0: Not a Combination Product 01/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/29/2024 Labeler - Shenzhen Xiaomai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen Xiaomai Manufacturing Co., Ltd. 712999147 manufacture(83872-012)