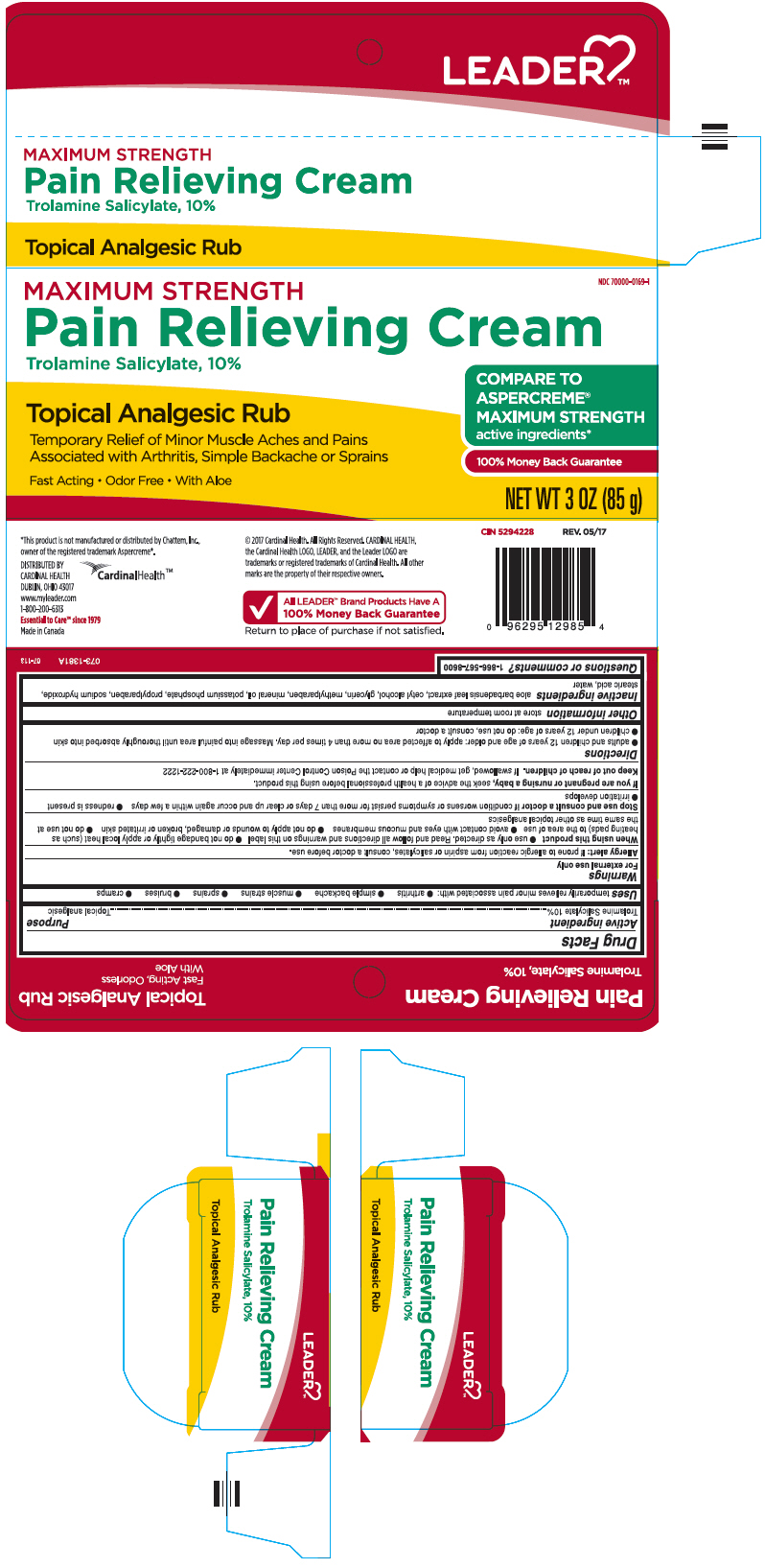
Label: LEADER MAXIMUM STRENGTH PAIN RELIEVING ANALGESIC- trolamine salicylate cream
- NDC Code(s): 70000-0169-1
- Packager: Cardinal Health, 110 dba LEADER
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert!
If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- ♦
- use only as directed. Read an follow all directions and warnings on this label
- ♦
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- ♦
- avoid contact with eyes and mucous membranes
- ♦
- do not apply to wounds or damaged, broken or irritated skin
- ♦
- do not use at the same time as other topical analgesics
Stop use and consult a doctor if condition worsens or symptoms persist for more than 7 days or clear up and occur again within a few days
- ♦
- redness is present
- ♦
- irritation develops
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
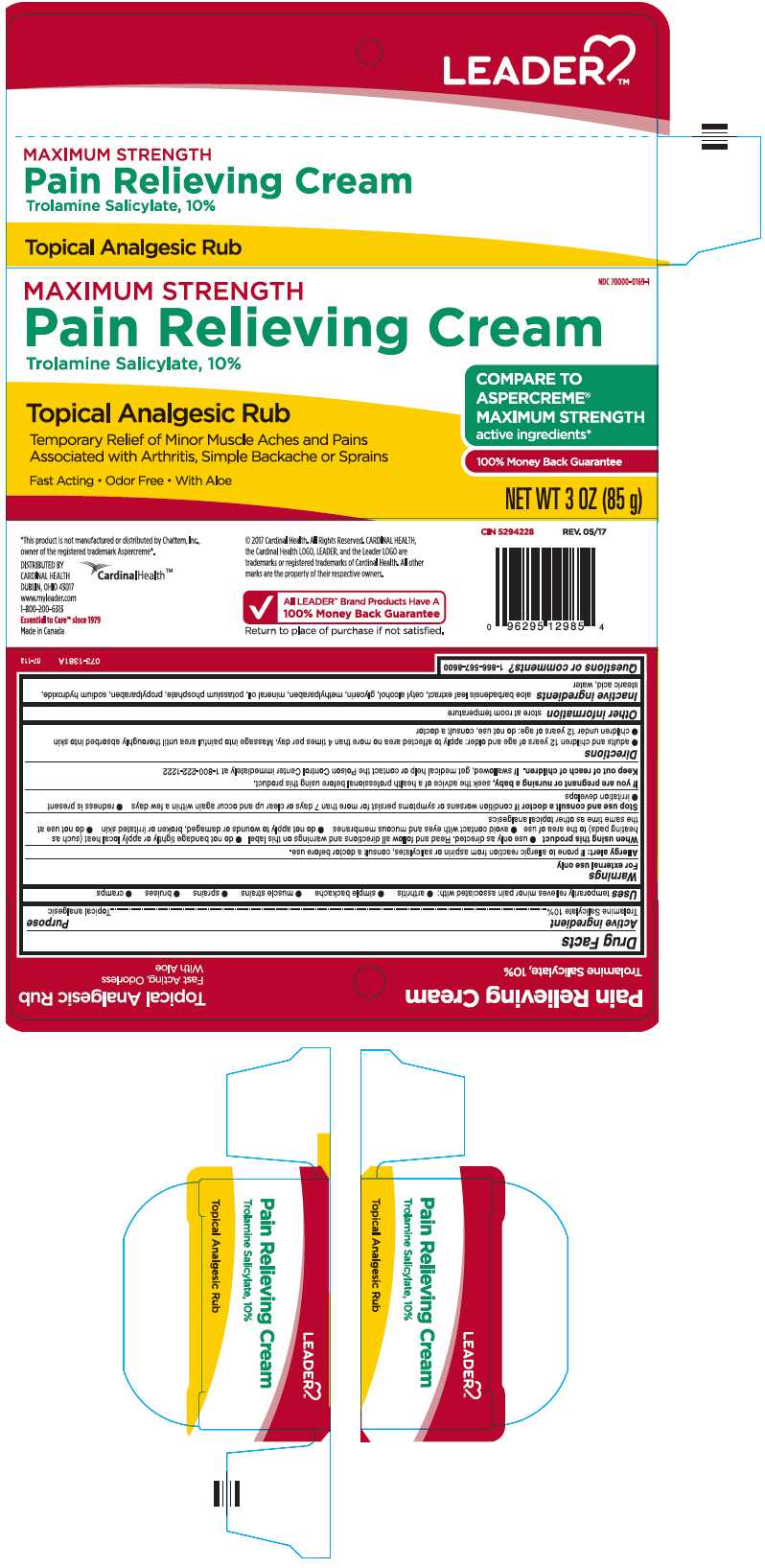
PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
MAXIMUM STRENGTH
NDC 70000-0169-1
Pain Relieving Cream
Trolamine Salicylate, 10%Topical Analgesic Rub
Temporary Relief of Minor Muscle Aches and Pains
Associated with Arthritis, Simple Backache or SprainsFast Acting • Odor Free • With Aloe
COMPARE TO
ASPERCREME®
MAXIMUM STRENGTH
active ingredients*100% Money Back Guarantee
NET WT 3 OZ (85 g)
-
INGREDIENTS AND APPEARANCE
LEADER MAXIMUM STRENGTH PAIN RELIEVING ANALGESIC
trolamine salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0169 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trolamine Salicylate (UNII: H8O4040BHD) (SALICYLIC ACID - UNII:O414PZ4LPZ) Trolamine Salicylate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Cetyl Alcohol (UNII: 936JST6JCN) Glycerin (UNII: PDC6A3C0OX) Methylparaben (UNII: A2I8C7HI9T) Mineral Oil (UNII: T5L8T28FGP) Potassium Phosphate, Unspecified Form (UNII: B7862WZ632) Propylparaben (UNII: Z8IX2SC1OH) Sodium Hydroxide (UNII: 55X04QC32I) Stearic Acid (UNII: 4ELV7Z65AP) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0169-1 1 in 1 CARTON 08/31/2017 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 08/31/2017 Labeler - Cardinal Health, 110 dba LEADER (063997360) Registrant - Garcoa, Inc (036464697) Establishment Name Address ID/FEI Business Operations Garcoa, Inc 036464697 MANUFACTURE(70000-0169)