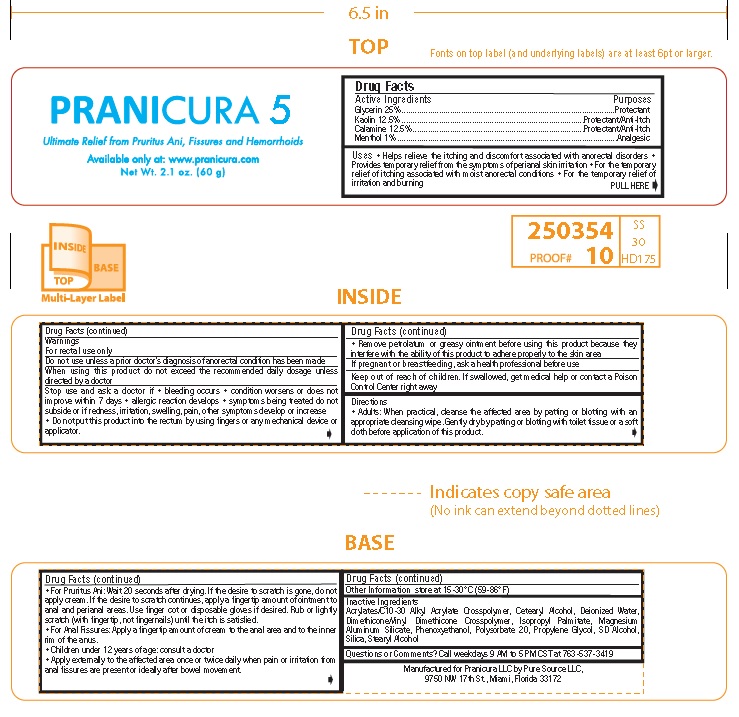
Label: PRANICURA 5- glycerin, kaolin, calamine, menthol cream
- NDC Code(s): 67676-007-01
- Packager: Sarati International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For rectal use only
Do not useunless a prior doctor’s diagnosis of anorectal condition has been made
When using this productdo not exceed the recommended daily dosage unless
directed by a doctor
Stop use and ask a doctor if• bleeding occurs • condition worsens or does not
improve within 7 days • allergic reaction develops • symptoms being treated do not
subside or if redness, irritation, swelling, pain, other symptoms develop or increase
• Do not put this product into the rectum by using fingers or any mechanical device or
applicator.• Remove petrolatum or greasy ointment before using this product because they
interfere with the ability of this product to adhere properly to the skin area
If pregnant or breastfeeding, ask a health professional before use - KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRANICURA 5
glycerin, kaolin, calamine, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67676-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 25 g in 100 g KAOLIN (UNII: 24H4NWX5CO) (KAOLIN - UNII:24H4NWX5CO) KAOLIN 12.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 12.5 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) WATER (UNII: 059QF0KO0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67676-007-01 60 g in 1 JAR; Type 0: Not a Combination Product 10/18/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 10/18/2021 Labeler - Sarati International, Inc. (160219770)