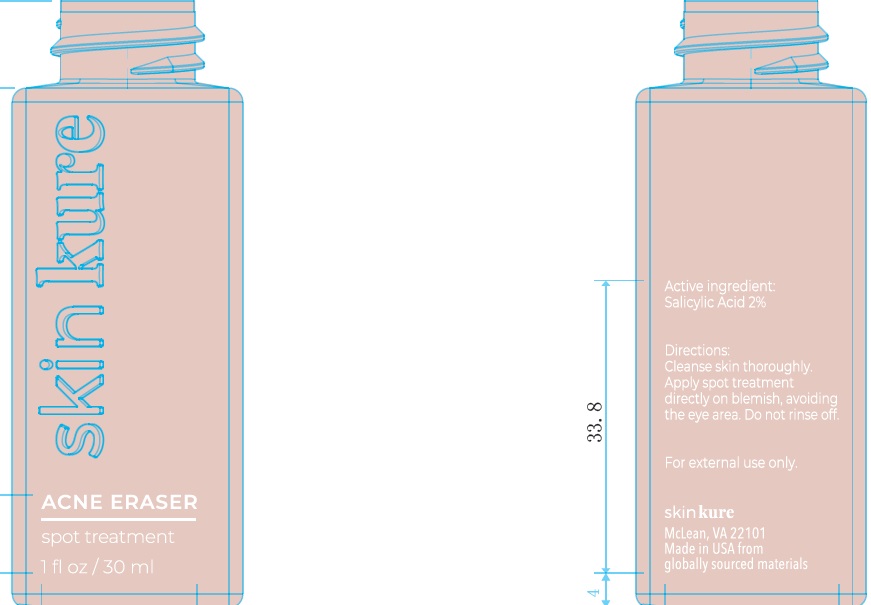
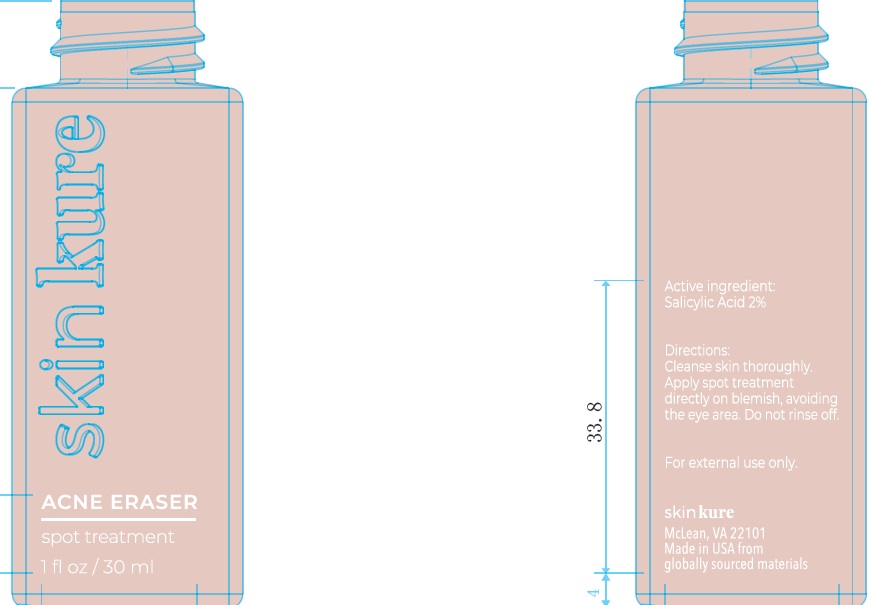
Label: SKINKURE ACNE ERASER SPOT TREATMENT- salicylic acid gel
- NDC Code(s): 83865-100-10
- Packager: SKINKURE, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses:
- Warnings:
- Directions:
-
Inactive Ingredients:
Water, SD Alcohol 40-B (Alcohol Denat), Propanediol, Glycerin, Glycolic Acid, Mandelic Acid, Ammonium Acryloyldimethyltaurate/VPCopolymer, Niacinamide, SodiumHydroxide, 1,2-Hexanediol, Caprylyl Glycol, Morinda Citrifolia Extract, Melaleuca Alternifolia (Tea Tree) Flower/Leaf/Stem Extract, Arginine, Opuntia Ficus-Indica Stem Extract, Centella Asiatica Extract, Brassica Juncea Sprout Extract, Brassica Oleracea Botrytis Extract, Brassica Oleracea Capitata (Cabbage) Sprout Extract, Brassica Oleracea Gemmifera Extract, Wasabia Japonica Root Extract, Brassica Oleracea Italica (Broccoli) SproutExtract, Brassica Oleracea Acephala Sprout Extract, Dextrin, Polydextrose, Amylopectin, Garcinia Mangostana Fruit Extract, Withania Somnifera Root Extract, Foeniculum Vulgare (Fennel) Seed Extract
- Questions or comments?
- 83865-100
-
INGREDIENTS AND APPEARANCE
SKINKURE ACNE ERASER SPOT TREATMENT
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83865-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) GLYCOLIC ACID (UNII: 0WT12SX38S) MANDELIC ACID (UNII: NH496X0UJX) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MORINDA CITRIFOLIA LEAF (UNII: 7UOL7P5FF5) MELALEUCA ALTERNIFOLIA FLOWERING TOP (UNII: 5AZ4S6N66F) ARGININE (UNII: 94ZLA3W45F) OPUNTIA FICUS-INDICA STEM (UNII: MUD8892KHL) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) WASABI ROOT (UNII: 69JM5DKE22) BROCCOLI SPROUT (UNII: 128UH9LOAE) POLYDEXTROSE (UNII: VH2XOU12IE) GARCINIA MANGOSTANA FRUIT (UNII: 832X5KK78Y) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) FENNEL SEED (UNII: G3QC02NIE6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83865-100-10 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/15/2024 Labeler - SKINKURE, LLC (003445854)