Label: CVS HEALTH PAIN RELIEF- menthol, unspecified form gel
- NDC Code(s): 66902-920-03, 66902-920-04
- Packager: NATURAL ESSENTIALS, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 19, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
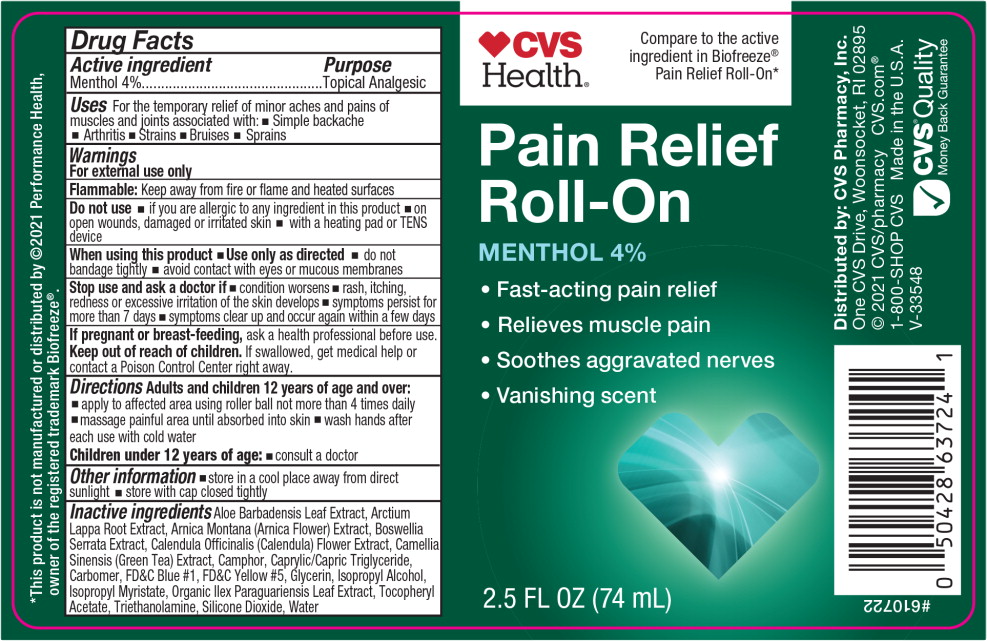
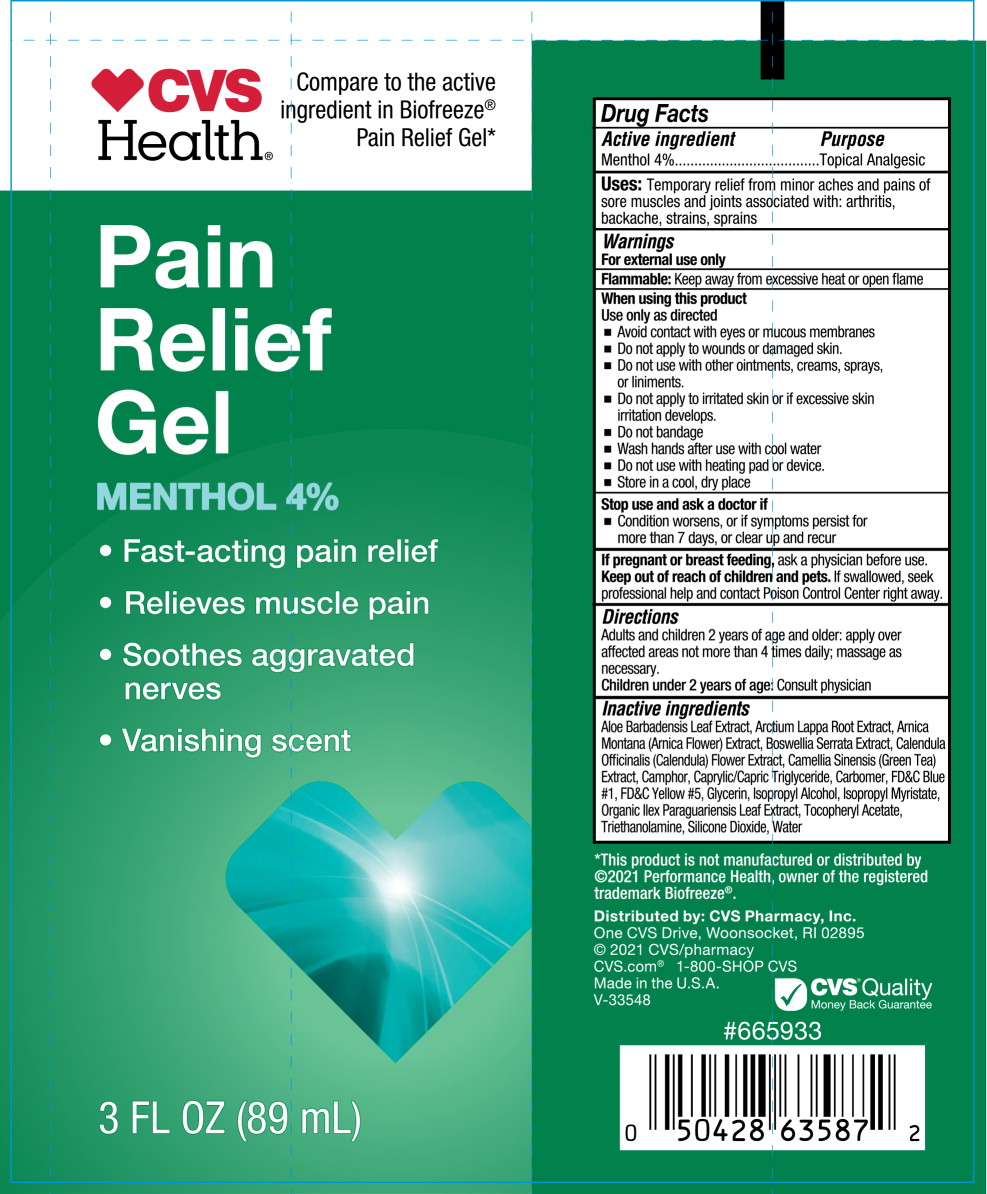
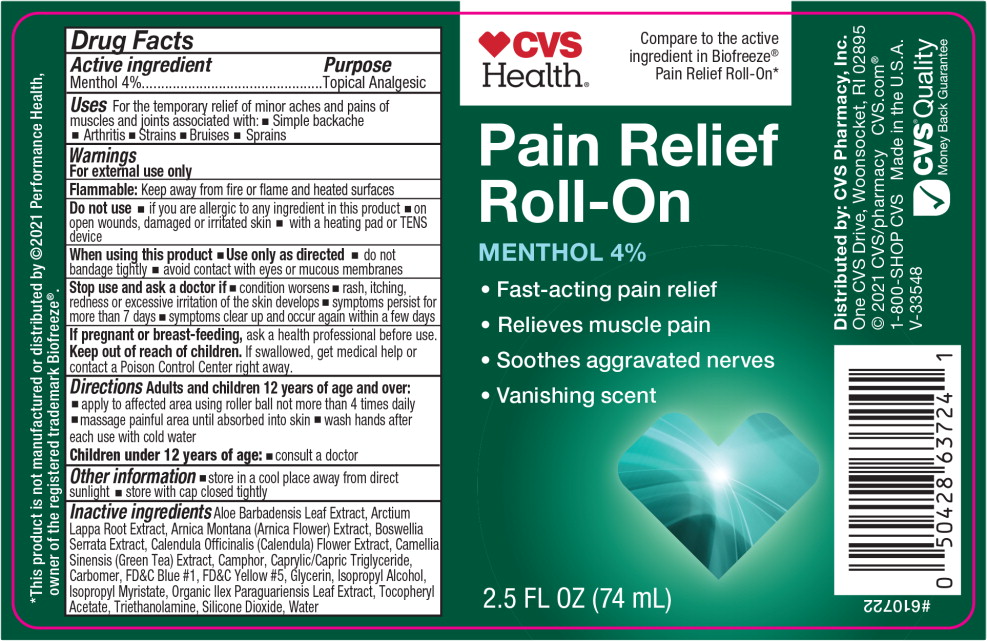
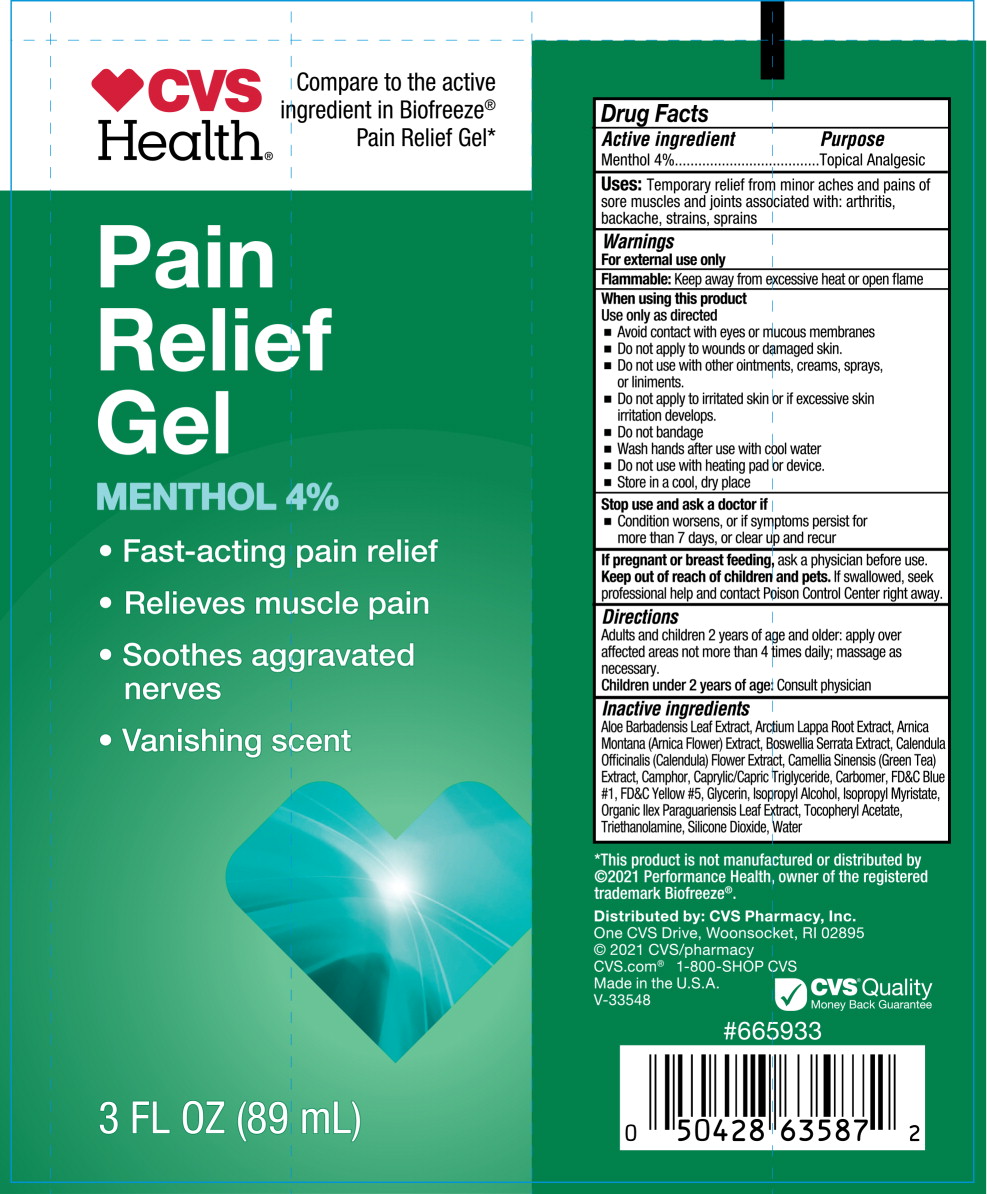
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Flammable: Keep away from fire or flame and heated surfaces
Do not use
- if you are allergic to any ingredient in this product
- on open wounds, damaged or irritated skin
- with a heating pad or TENS device
When using this product
- Use only as directed
- do not bandage tightly
- avoid contact with eyes or mucous membranes
- Directions
- Other information
-
Inactive ingredients
Aloe Barbadensis Leaf Extract, Arctium Lappa Root Extract, Arnica Montana (Arnica Flower) Extract, Boswellia Serrata Extract, Calendula Officinalis (Calendula) Flower Extract, Camellia Sinensis (Green Tea) Extract, Camphor, Caprylic/Capric Triglyceride, Carbomer, FD&C Blue #1, FD&C Yellow #5, Glycerin, Isopropyl Alcohol, Isopropyl Myristate, Organic Ilex Paraguariensis Leaf Extract, Tocopheryl Acetate, Triethanolamine, Silicone Dioxide, Water
- Principal Display Panel - 74 mL Bottle Label
- Principal Display Panel - 89 mL Tube Label
-
INGREDIENTS AND APPEARANCE
CVS HEALTH PAIN RELIEF
menthol, unspecified form gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66902-920 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL ALCOHOL (UNII: ND2M416302) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66902-920-03 74 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2021 2 NDC:66902-920-04 89 mL in 1 TUBE; Type 0: Not a Combination Product 01/20/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/20/2021 Labeler - NATURAL ESSENTIALS, INC. (947484713) Establishment Name Address ID/FEI Business Operations NATURAL ESSENTIALS, INC. 947484713 MANUFACTURE(66902-920)